Method for producing 2,6-dichloro benzal chloride with byproduct of 2,6-dichlorotoluene
A technology of dichlorobenzylidene dichloride and dichlorotoluene is applied in the production field of chemical raw materials, can solve the problems of single component, unfavorable reaction and high cost, and achieves flexible operation control, improved economic benefit and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
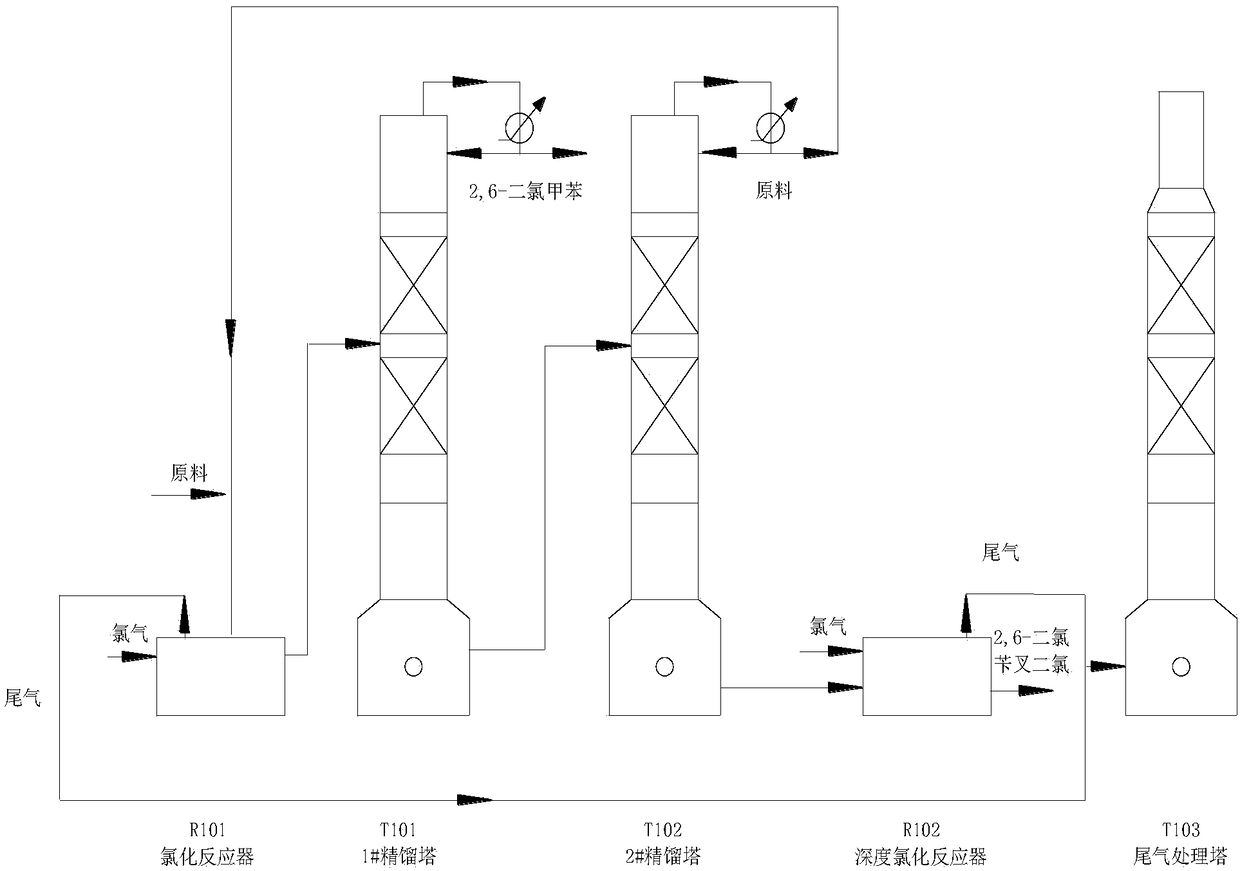
[0026] Such as figure 1 In the process flow shown, a 2000mL tank reactor is coupled with a rectification tower, 2000g of 2-chloro-6-nitrotoluene is added, and 20g of catalyst tetramethylammonium chloride is added. During chlorination, the reactor chlorine gas flow rate is 300mL / min, and the chlorination temperature is 150°C. The operating pressure of No. 1 rectifying tower is 2.5kPa, the temperature at the top of the tower is 85°C, and the temperature at the bottom of the tower is 135°C. The packing height of the rectification column is 1m, and the column diameter is 30mm. Reactive distillation yielded 469.40 g of 2,6-dichlorotoluene with a purity of 99%, and deep chlorination gave 1903.41 g of 2,6-dichlorobenzylidene dichloride with a purity of 95.0%.
Embodiment 2
[0028] Such as figure 1 As shown in the process flow, a 000mL tank reactor is coupled with a rectification tower, 2000g of 2-chloro-6-nitrotoluene is added, and 20g of catalyst tetraethylammonium chloride is added. During chlorination, the chlorine gas flow rate in the reactor is 300mL / min, and the chlorination temperature is 160°C. The operating pressure of No. 1 rectifying tower is 2.5kPa, the temperature at the top of the tower is 85°C, and the temperature at the bottom of the tower is 135°C. The packing height of the rectification column is 1m, and the column diameter is 30mm. Reactive distillation yielded 512.08 g of 2,6-dichlorotoluene with a purity of 99%, and deep chlorination gave 1848.88 g of 2,6-dichlorobenzylidene dichloride with a purity of 95.5%.
Embodiment 3
[0030] Such as figure 1 In the process flow shown, a 2000mL photochemical reactor is coupled with a rectification tower, 2000g of 2-chloro-6-nitrotoluene is added into the reactor, and 20g of catalyst tetramethylammonium chloride is added. During chlorination, the chlorine flow rate of each reactor is 300mL / min, and the chlorination temperature is 50°C. The operating pressure of No. 1 rectifying tower is 2.5kPa, the temperature at the top of the tower is 85°C, and the temperature at the bottom of the tower is 135°C. The packing height of the rectification column is 1.2m, and the column diameter is 30mm. Reactive distillation yielded 560.01 g of 2,6-dichlorotoluene with a purity of 99%, and deep chlorination gave 1,796.17 g of 2,6-dichlorobenzylidene dichloride with a purity of 97.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com