Targeting Muc1 type chimeric antigen receptor modified T cell and application thereof
A technology of chimeric antigen receptors and intracellular domains, applied in the fields of genetic engineering and oncology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
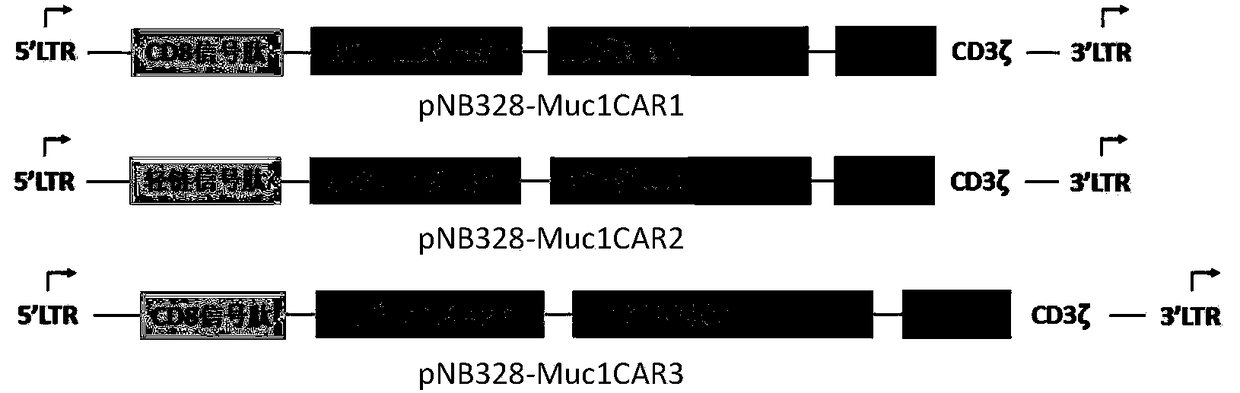
[0086] Example 1: Construction of recombinant plasmids pNB328-Muc1CAR1, pNB328-Muc1CAR2, pNB328-Muc1CAR3 and the acquisition of Muc1CAR gene-modified T cells
[0087] 1. Artificial synthesis containing signal peptide (CD8 signal peptide or light chain signal peptide), antigen recognition single-chain antibody (anti-Muc1scFv), hinge region (CD8 hinge region or IgG4Fc CH2CH3 hinge region), CD8 transmembrane region, costimulatory signal The Muc1CAR exogenous genes of the molecular intracellular domain and the immunoreceptor tyrosine activation motif (named Muc1CAR1, Muc1CAR2 and Muc1CAR3, respectively), and introduced a multicloning restriction site (BglII-XbaI-EcoRI-BamHI ), insert a restriction site (SalI-NheI-HindIII-SpeI) downstream of it, entrust Shanghai Jierui Biology Co., Ltd. to synthesize it, and put it into the pNB328-EF1α vector (containing EF1α promoter pNB328, see CN 201510812654.9 for the pNB328 vector) to form recombinant plasmids, named pNB328-Muc1CAR1, pNB32...
Embodiment 2
[0091] Example 2: The expression of T cells after PBMCs cells derived from different patients were activated by Muc1CAR exogenous gene modification Muc1CAR gene expression identification
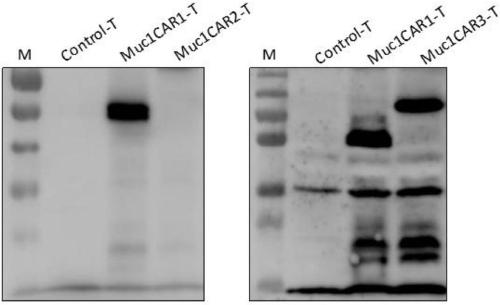
[0092] The activated T cells of different patients after the recombinant plasmid pNB328-Muc1CAR1 or pNB328-Muc1CAR2 or pNB328-Muc1CAR3 were electroporated by 2×10 6 Cell number Collect the cell pellet, add 80ul of 2X SDS-PAGE Loadingbuffer, boil at 100°C for 10min, and store at -20°C for later use. Western blotting (using CD3ζ antibody as the primary antibody, and HRP-goat anti-human secondary antibody) was used to detect the expression of Muc1CAR.
[0093] The result is as figure 2 shown. The results showed that T cells could stably express Muc1CAR1 and Muc1CAR3 recombinant proteins, but could not express Muc1CAR2 recombinant proteins.
Embodiment 3
[0094] Example 3: PBMCs cells from different patients were modified by Muc1CAR exogenous gene in T cell genome Detection of expression level of Muc1CAR genome
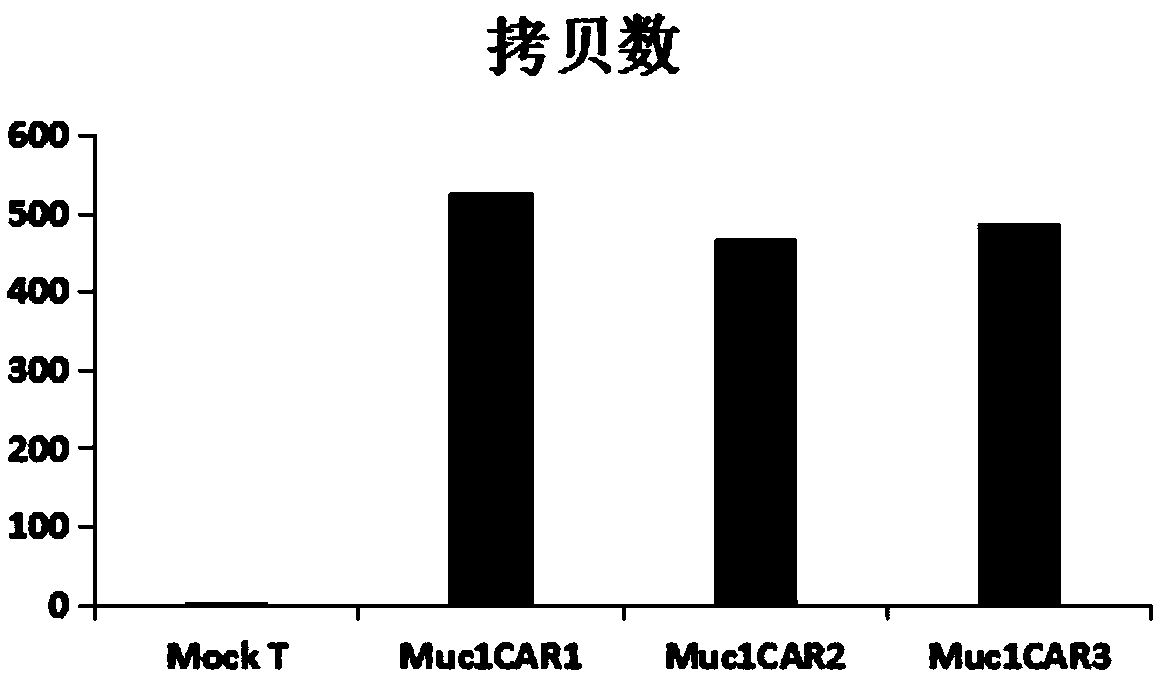
[0095] Genomic DNA of Mock T cells (T cells electroporated with pNB328 empty vector) and Muc1CAR-T cells (kit method) was extracted, and the experimental steps were determined according to the instructions attached to the kit, and the DNA concentrations of Mock T cells and Muc1CAR-T cells were measured. The expression level of the Muc1CAR genome was detected by real-time fluorescent quantitative PCR. The reaction program was: 50°C, 2min→95°C, 10min→95°C, 15s→60°C, 1min, 40 cycles.
[0096] The result is as image 3 shown. The results showed that the Muc1CAR1, Muc1CAR2 and Muc1CAR3 genomes were all integrated into the T cell genome through the PB transposase system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com