Solid-phase fragment synthesis method of plecanatide
A synthetic method, the technology of Prica, is applied in the field of polypeptide drug preparation, which can solve the problems of strong hydrophobicity, difficult coupling, and low coupling efficiency, and achieve the effects of less by-products, beneficial purification, and avoiding low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0092] Example 1: Synthesis of Fmoc-Leu-CTC resin with a substitution degree of 0.30 mmol / g.
[0093] Weigh 20.0g of 2-CTC resin with a substitution degree of 0.50mmol / g, add it to a solid-phase reaction column, wash it once with DMF, swell the resin with DCM for 30 minutes, and wash it three times with DMF, and take 10.6g of Fmoc-Leu- OH was dissolved in DCM, activated by adding 9.9ml DIEA in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours, washed 3 times with DMF, and sealed with DIEA:MeOH:DCM = 1:10:20 mixture Overnight, DCM and methanol were alternately washed, shrunk and dried to obtain Fmoc-Leu-CTC resin with a detection degree of substitution of 0.30 mmol / g.
Example Embodiment
[0094] Example 2: Synthesis of Fmoc-Val-CTC resin with a substitution degree of 0.50 mmol / g.
[0095] Weigh 62.5g of 2-CTC resin with a substitution degree of 0.80mmol / g, add it to a solid-phase reaction column, wash it once with DMF, swell the resin with DCM for 30 minutes, and wash it three times with DMF, and take 50.9g of Fmoc-Val- OH was dissolved in DCM, activated by adding 49.6ml DIEA in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours, washed 3 times with DMF, and sealed with a mixture of DIEA:MeOH:DMF=1:10:20 Overnight, DCM and MeOH were alternately washed, shrunk and dried to obtain Fmoc-Val-CTC resin with a detection substitution degree of 0.50 mmol / g.
Example Embodiment
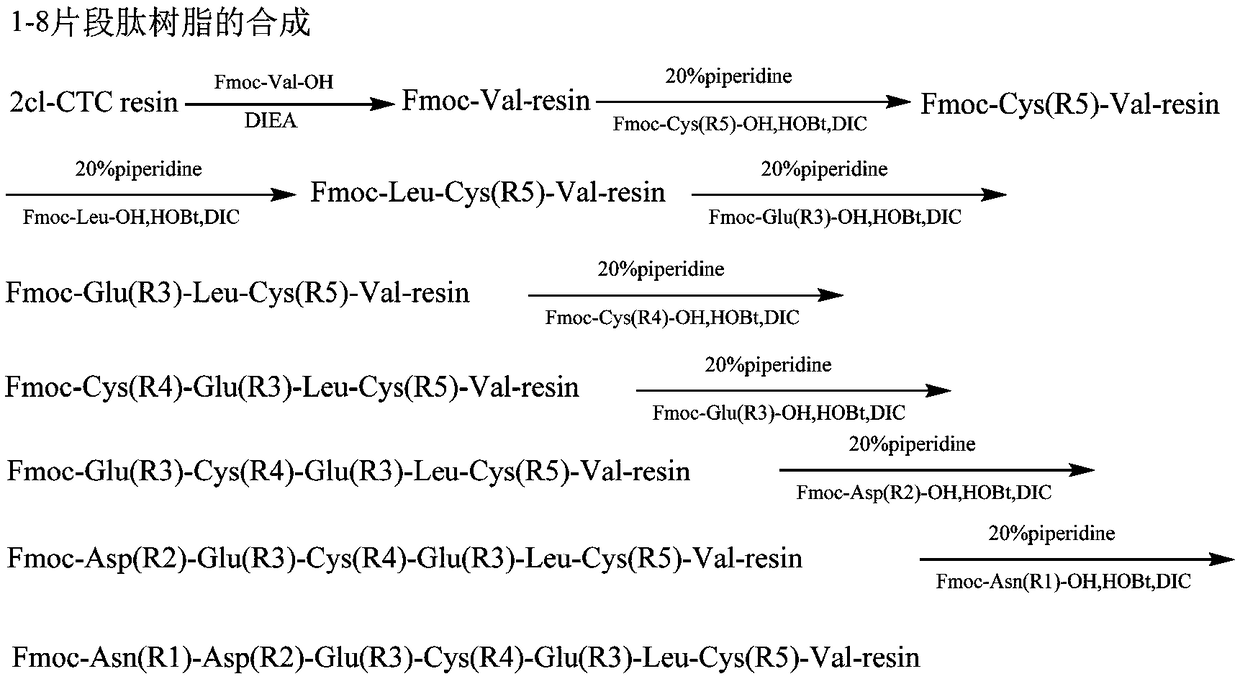
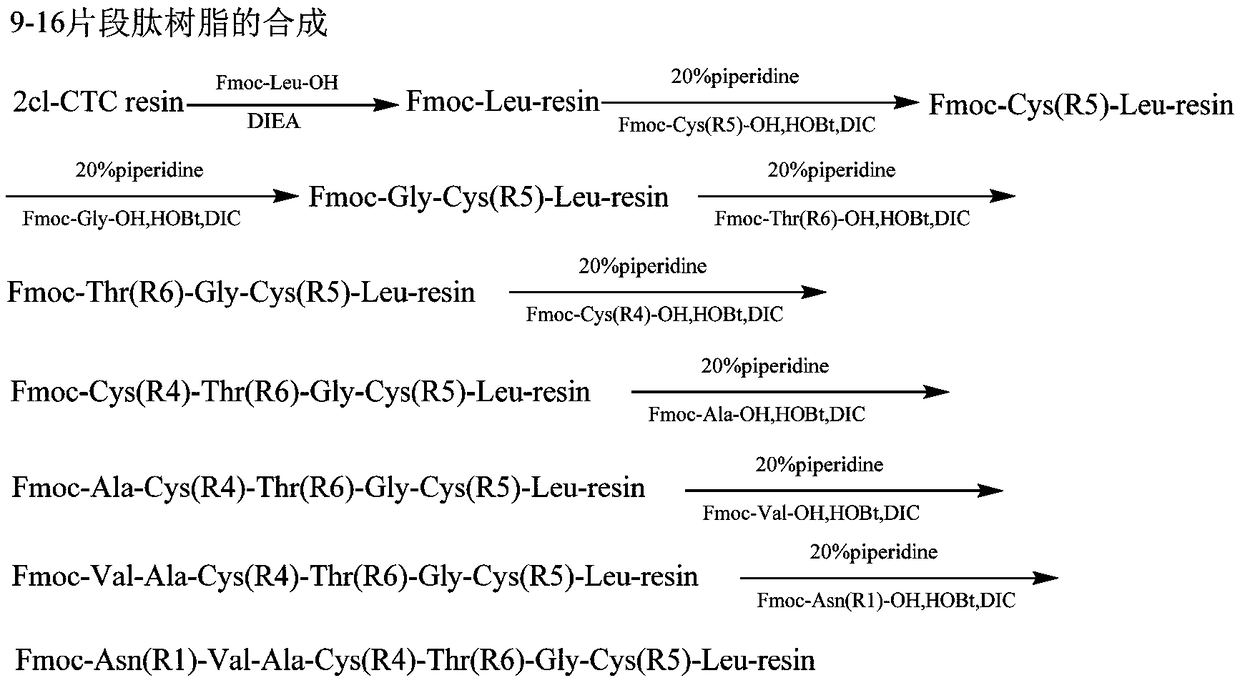
[0096] Implementation Case 3: Preparation of two groups of 8-peptides of pulikanatide
[0097] Weigh 10.0g (3mmol) Fmoc-Leu-CTC resin with a degree of substitution of 0.30mmol / g, add it to the solid-phase reaction column, wash once with DMF, and swell the Fmoc-Leu-CTC resin with DCM for 30 minutes, then use DMF: The mixed solution with piperidine volume ratio 4:1 removes Fmoc protection, and then washes with DMF 6 times, weighs 3.7g Fmoc-Cys(Acm)-OH, 1.2g HOBt, adds DMF solution to dissolve, and adds 1.4ml DIC under ice-water bath to activate, Add it to the above-mentioned reaction column filled with resin, react at room temperature for 2 hours, use ninhydrin to detect and judge the end of the reaction, if the resin is colorless and transparent, it means the reaction is complete; if the resin develops color, the reaction is incomplete, and you need to continue the reaction Re-dosing in 1 hour or a single dose or changing the condensation reagent. Ninhydrin detection is suitab...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap