Substituted pyridine compound and application thereof
A compound, pyridine technology, applied in the field of substituted pyridine compounds, can solve the problems of phase separation of mixed host materials, complex device preparation, etc., achieve high triplet energy level and glass transition temperature, improve transport performance, and reduce conjugation degree Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] The preparation methods of the compounds disclosed in the present invention are provided below. However, the present disclosure is not intended to be limited to any of the methods described herein. One skilled in the art can readily modify the described methods or utilize a different method to prepare one or more of the disclosed compounds. The following aspects are exemplary only, and are not intended to limit the scope of the present disclosure. Temperature, catalyst, concentration, reactant composition, and other process conditions may vary, and one skilled in the art of this disclosure can readily select appropriate reactants and conditions for a desired complex.
[0103] The meaning of the abbreviations in the embodiments of the present invention: PE: petroleum ether; DCM: dichloromethane; EA: ethyl acetate; DMSO-d6, deuterated dimethyl sulfoxide; CDCl3, deuterated chloroform; MeTHF: methyl tetrahydrofuran; Pb(dba) 2 : Tris(dibenzylideneacetone)dipalladium; S-Ph...
Embodiment 1
[0104] Embodiment 1: the synthesis of H-1 compound
[0105] Step 1: Synthesis of 9-(3-methyl-5-bromopyridin-2-yl)-9H-pyridin[2,3-b]indole
[0106] 9-(3-methyl-5-bromopyridin-2-yl)-9H-pyridin[2,3-b]indole is synthesized by the following reaction scheme 1:
[0107]
[0108] 2,5-dibromo-3-methylpyridine (2.51g, 10.0mmol), carboline (1.82g, 11.0mmol), CuI (381mg, 2.0mmol), 1-methylimidazole (246mg, 3.0mmol) , potassium carbonate (2.76g, 20.0mmol), and toluene (25mL) were placed in a 38mL sealed tube. After bubbling nitrogen for 5min, the temperature was raised to 120°C, and the reaction was refluxed for 48h. After the reaction was completed, filter and rinse the filter cake with ethyl acetate. The obtained filtrate was distilled under reduced pressure to remove the solvent, and then the resultant was purified through a silica gel column (petroleum ether: ethyl acetate = 10:1 → 5:1) to obtain a white solid 9-(3-methyl-5-bromo Pyridin-2-yl)-9H-pyridin[2,3-b]indole (1.75 g, 52%...
Embodiment 2
[0113] Embodiment 2: the synthesis of H-2 compound
[0114] The H-2 compound is synthesized by the following reaction formula 3
[0115]
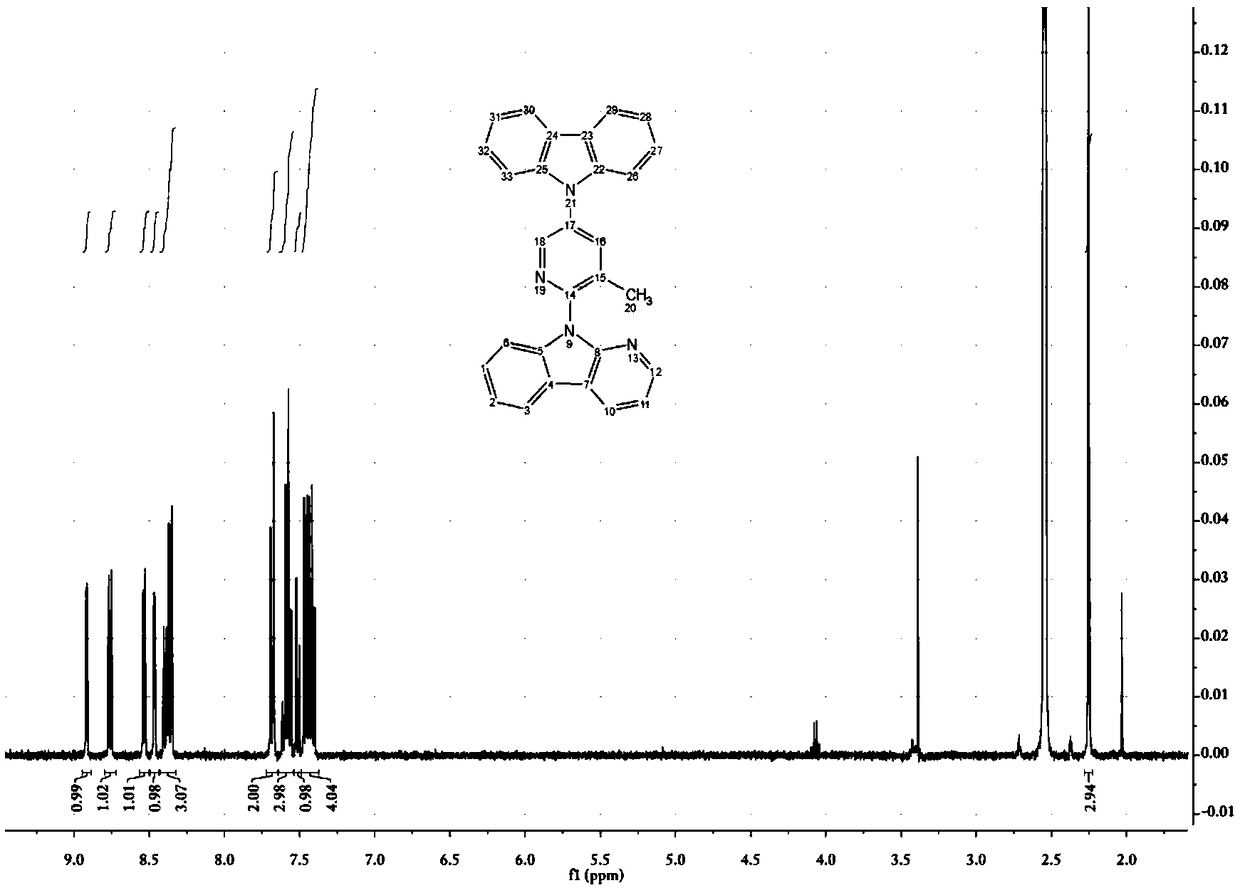
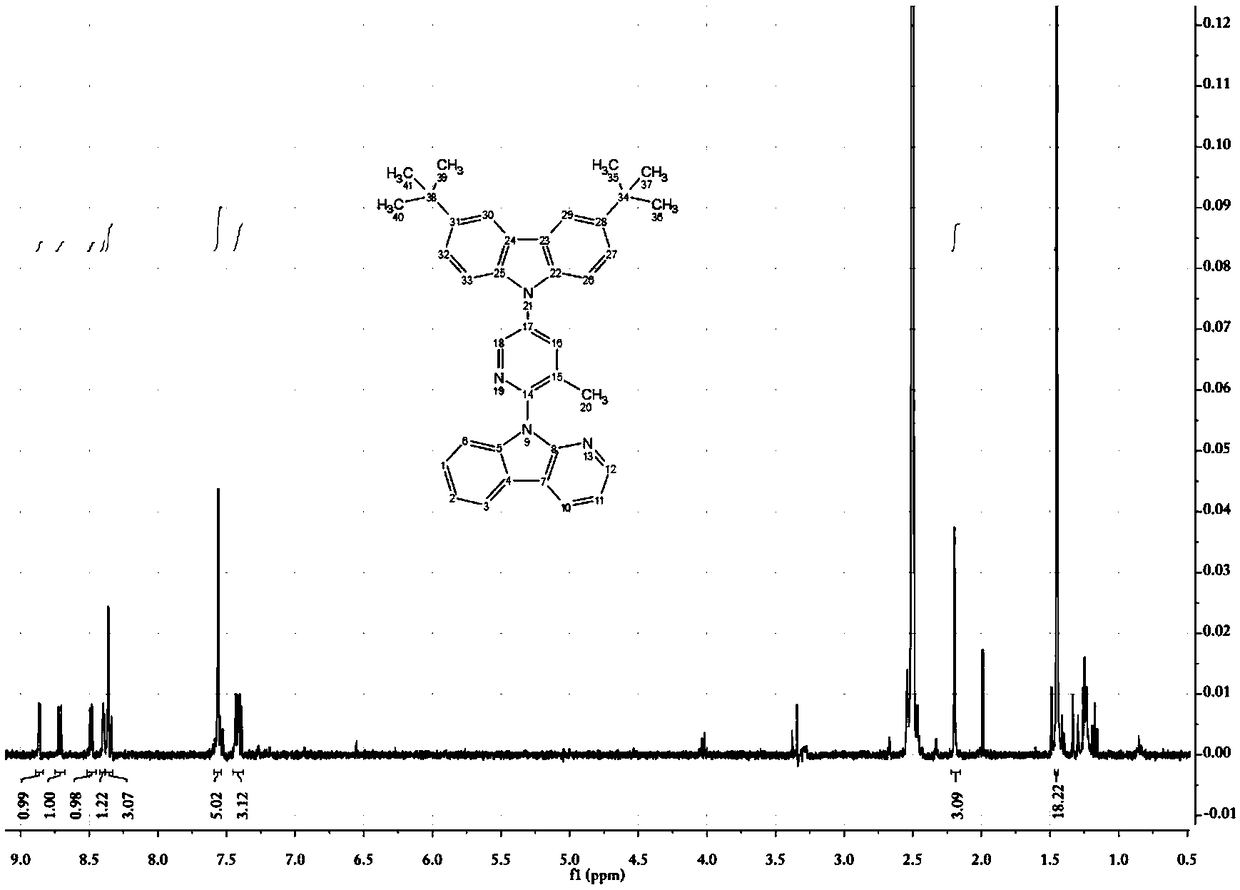
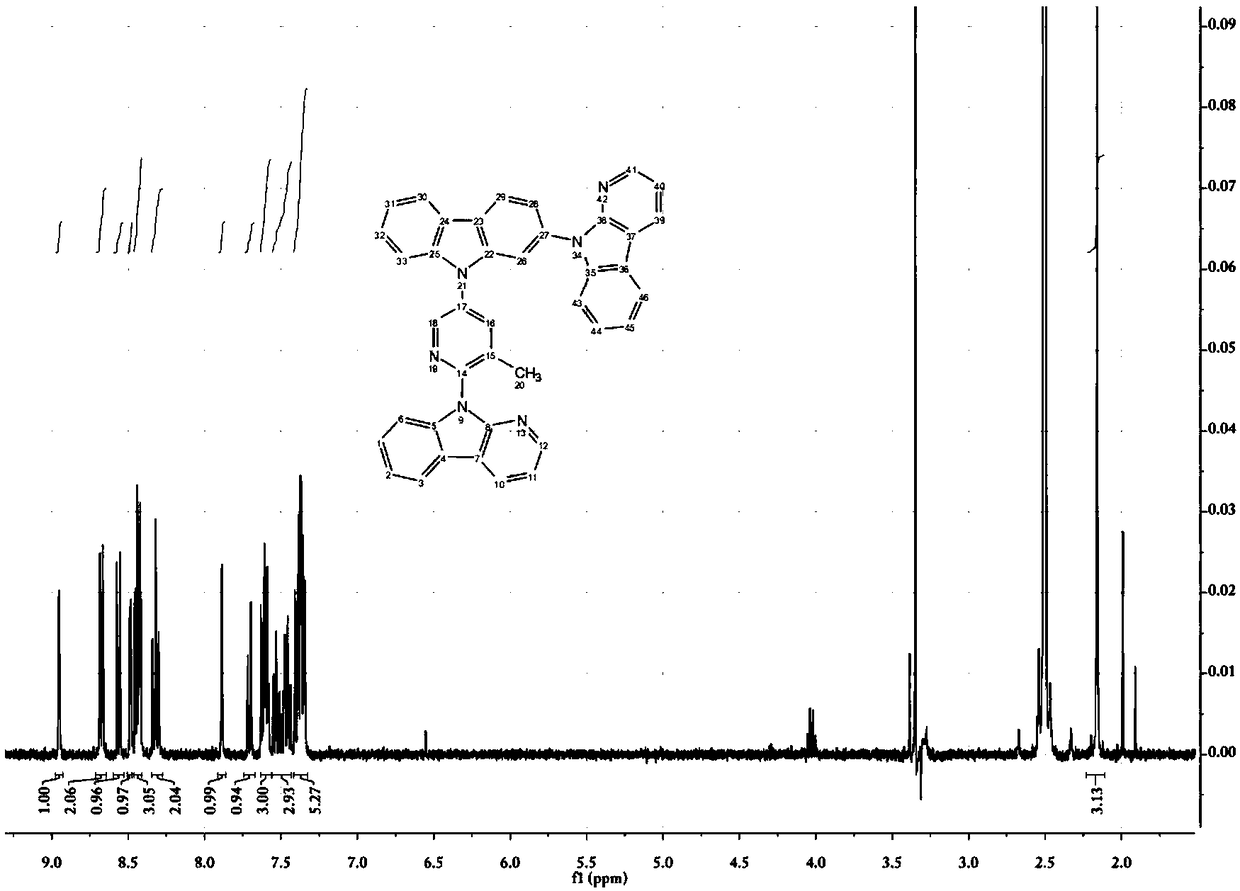
[0116] 9-(3-methyl-5-bromopyridin-2-yl)-9H-pyridin[2,3-b]indole (109mg, 0.32mmol), 3,6-di-tert-butylcarbazole (99mg , 0.35mmol), Pb (dba) 2 (59mg, 0.06mmol), S-Phos (26mg, 0.06mmol), sodium tert-butoxide (61mg, 0.64mmol), and toluene (3mL) were placed in a 38mL sealed tube, heated to 120°C after nitrogen bubbling for 5min, and reacted Reflux for 28h. After the reaction was completed, it was filtered with celite, and the filter cake was rinsed with ethyl acetate. The obtained filtrate was evaporated under reduced pressure to remove the solvent, and then the resultant was purified by silica gel column (petroleum ether: ethyl acetate = 10:1) to obtain a light yellow solid (158 mg, yield 92%). 1 H-NMR (400MHz, DMSO-d 6 )δ: 8.86 (d, J = 2.7Hz, 1H), 8.71 (dd, J = 7.7, 1.6Hz, 1H), 8.48 (dd, J = 4.8, 1.6Hz, 1H), 8.42–8.38 (m, 1H ),8.38–8.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com