Method for synthesizing di-tert-butylphosphine biphenyl compound
A technology of di-tert-butylphosphine biphenyl and di-tert-butylphosphine, which is applied in the field of organic synthesis, can solve the problems of affecting the reaction yield, difficulty in post-processing, and high equipment requirements, so as to optimize the reaction process, avoid the use of catalysts, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
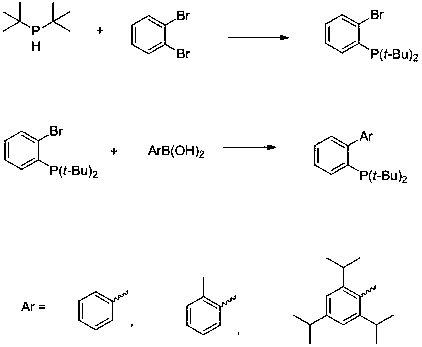
[0016] Example 1: Synthesis of 2-(di-tert-butylphosphine)biphenyl
[0017] Under the protection of argon, add reaction solvent 1L toluene to dry reactor, then add di-tert-butylphosphine (146 g, 1 mol), o-dibromobenzene (236 g, 1 mol), bis[di-tert-butyl (4-Dimethylaminophenyl) phosphine] palladium (0) (6.4 g, 0.01 mol) and sodium carbonate (424 g, 4 mol), then heated to 100 ºC for 6 hours, after the reaction solution cooled to 20-30 ºC , directly added phenylboronic acid (122 g, 1 mol) to the system, and then raised the temperature to 80 ºC for 10 hours; then added water to quench the reaction, extracted, dried, and distilled off the solvent under reduced pressure, then recrystallized in methanol to obtain 2- (Di-tert-butylphosphine)biphenyl 262g, yield 88%. 31 P NMR (121 MHz, CDCl 3 ) δ 18.7; 1 H NMR (300 MHz, CDCl 3 ) δ 7.98-7.86(m, 1H), 7.40-7.22 (m, 8H), 1.16 (d, 18H, J = 11.6 Hz).
Embodiment 2
[0018] Example 2: Synthesis of 2-(di-tert-butylphosphino)-2',4',6'-triisopropylbiphenyl
[0019] Under the protection of argon, add the reaction solvent 1L toluene to the dry reactor, then add di-tert-butylphosphine (146g, 1 mol), o-dibromobenzene (212 g, 0.9 mol), bis[di-tert-butyl (4-Dimethylaminophenyl)phosphine] palladium (0) (3.2g, 0.005 mol) and sodium carbonate (530 g, 5 mol), then heated to 80 ºC for 8 hours, and the temperature of the reaction solution was cooled to 20-30 ºC Finally, 2,4,6-triisopropylphenylboronic acid (273 g, 1.1 mol) was directly added to the system, and then heated to 100°C for 12 hours; then water was added to quench the reaction, extracted, dried, and evaporated under reduced pressure to remove Recrystallization in methanol after the solvent was used to obtain 364 g of 2-(di-tert-butylphosphino)-2',4',6'-triisopropylbiphenyl with a yield of 86%. 31 P NMR (121 MHz, C 6 D. 6 ) δ21.4; 1 H NMR (300 MHz, C 6 D. 6 ) δ 7.85-7.74 (m, 1H), 7.33-7.2...
Embodiment 3
[0020] Example 3: Synthesis of 2-(di-tert-butylphosphine)-2'-methylbiphenyl
[0021] Under the protection of argon, add the reaction solvent 1L toluene to the dry reactor, then add di-tert-butylphosphine (146g, 1 mol), o-dibromobenzene (212 g, 0.9 mol), bis[di-tert-butyl (4-Dimethylaminophenyl)phosphine] palladium (0) (3.2g, 0.005 mol) and sodium carbonate (530 g, 5 mol), then heated to 80 ºC for 6 hours, and the temperature of the reaction solution was cooled to 20-30 ºC After that, directly add 2-methylphenylboronic acid (185g, 1.1 mol) to the system, and then raise the temperature to 100 ºC for 10 hours; Crystallization gave 281 g of 2-(di-tert-butylphosphine)-2'-methylbiphenyl, with a yield of 90%. 31 P NMR (121 MHz, C 6 D. 6 ) δ 20.8; 1 H NMR (300 MHz, CDCl 3 ) δ 7.90-7.84 (m, 1H), 7.37-7.08 (m, 7H), 2.12 (s, 3H), 1.17 (d, J =11.5Hz, 9H), 1.16 (d, J =11.3 Hz, 9H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com