A kind of tripeptide and its preparation method and application
A peptide resin, amino acid technology, applied in the field of peptides, to achieve the effect of anti-aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
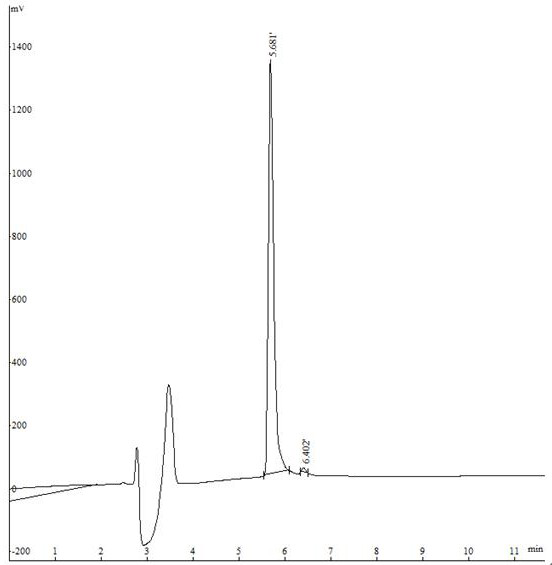
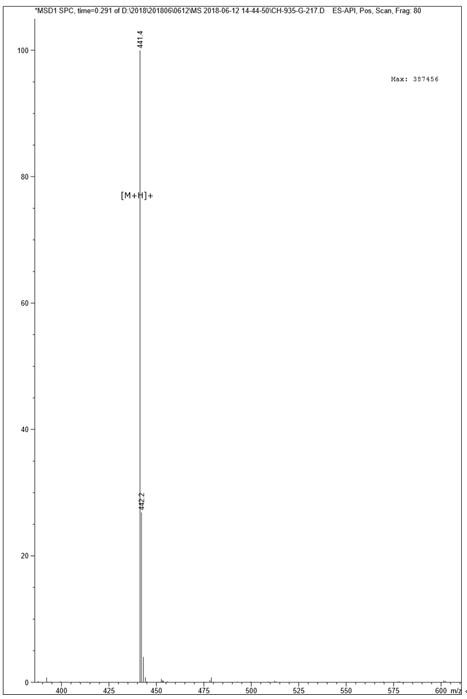
[0047] Example 1 Polypeptide Solid Phase Synthesis Synthetic Polypeptide FQF
[0048] Adopt the standard Fomc scheme, choose dichloro resin, according to the sequence characteristics of the amino acid sequence Phe-Gln-Phe, make the peptide chain extend from the C-terminus to the N-terminus one by one, use 20% piperidine / N,N-dimethyl for each step of condensation Formamide (DMF) solution (15mL / g) was treated for 15 minutes to remove the Fmoc protecting group; after that, detection was performed, the piperidine solution was removed, a dozen resins were taken, washed three times with ethanol, and ninhydrin, pyridine, and phenol were added. One drop, heated at 105°C-110°C for 5 minutes, turning dark blue is a positive reaction, you can continue to receive the next amino acid, if it does not change color, it is negative, and needs to be deprotected again. Each washing is successively washed twice with 15mLDMF, 15mL methanol, and 15mLDMF respectively. Condensate after the first cle...
Embodiment 2
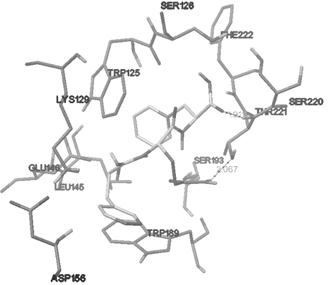
[0050] Example 2 Molecular docking evaluation of CD38 inhibitory activity of polypeptides
[0051] 1. Target receptor structure determination
[0052] It varies according to the way it captures or the small molecule substances it binds. By comparing the crystal structures in the protein database PDB, in view of the stability of the key residue Glu226 and the complex’s effect on NAD + For the simulation of the binding between the molecule and CD38, the co-crystal structure of CD38 and nicotinamide mononucleotide (NMN) was selected for molecular docking, and the PDBID was 3DZK.
[0053] 2. FQF Ligand Establishment
[0054] Chem3D software was used to draw the peptide FQF to generate a three-dimensional structure, and the structure was optimized by force field and saved for future use.
[0055] 3. Molecular docking
[0056] The crystal structure 3DZK of CD38 was downloaded from the PDB database, and the energy lattice calculation was performed on the A chain, and the determine...
Embodiment 3
[0057] Example 3 Synthetic polypeptide FQF in vitro antioxidant test
[0058] 1. Solution preparation
[0059] Preparation of 0.1mM and 0.5mM synthetic peptide (FQF) solutions: Accurately weigh 4.405mg of synthetic peptide, dissolve it in 10mL of medium, pass through a 0.22μm filter head, the concentration of the mother solution is 1mM, and then dilute the mother solution with the medium to the concentration required for the experiment.
[0060] h 2 o 2 Solution preparation: mix 30% hydrogen peroxide with DMEM to make a mother solution with a concentration of 2mmol / L, and then dilute to 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0 and 2.0 The solution
[0061] 2. Construction of hydrogen peroxide injury model
[0062] Hek293 cells were planted in a 96-well plate at a density of 10000 / well, and after 24 hours of complete attachment, each group was added with a concentration of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0 and 2.0mmol / L of hydrogen peroxide medium,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com