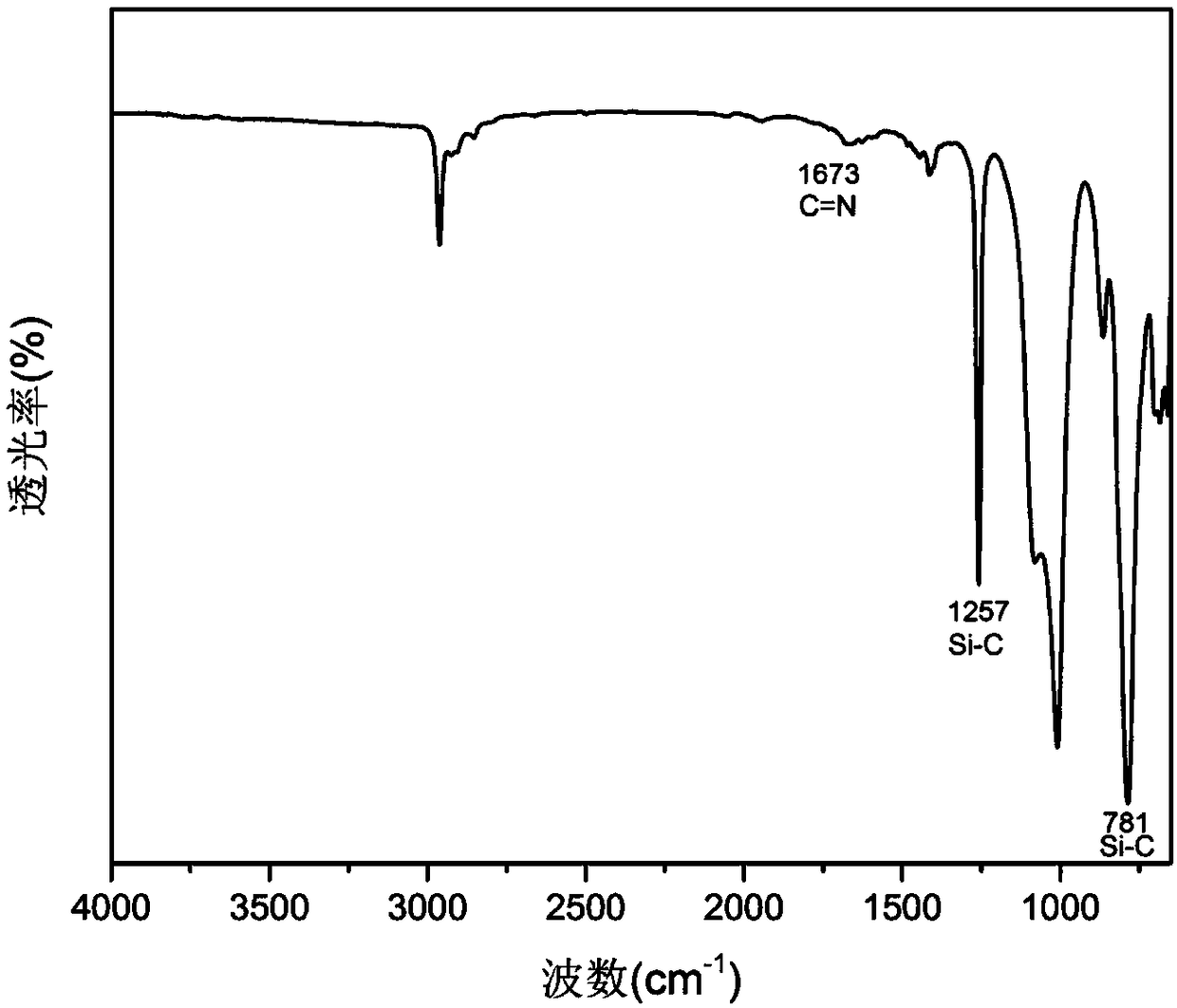
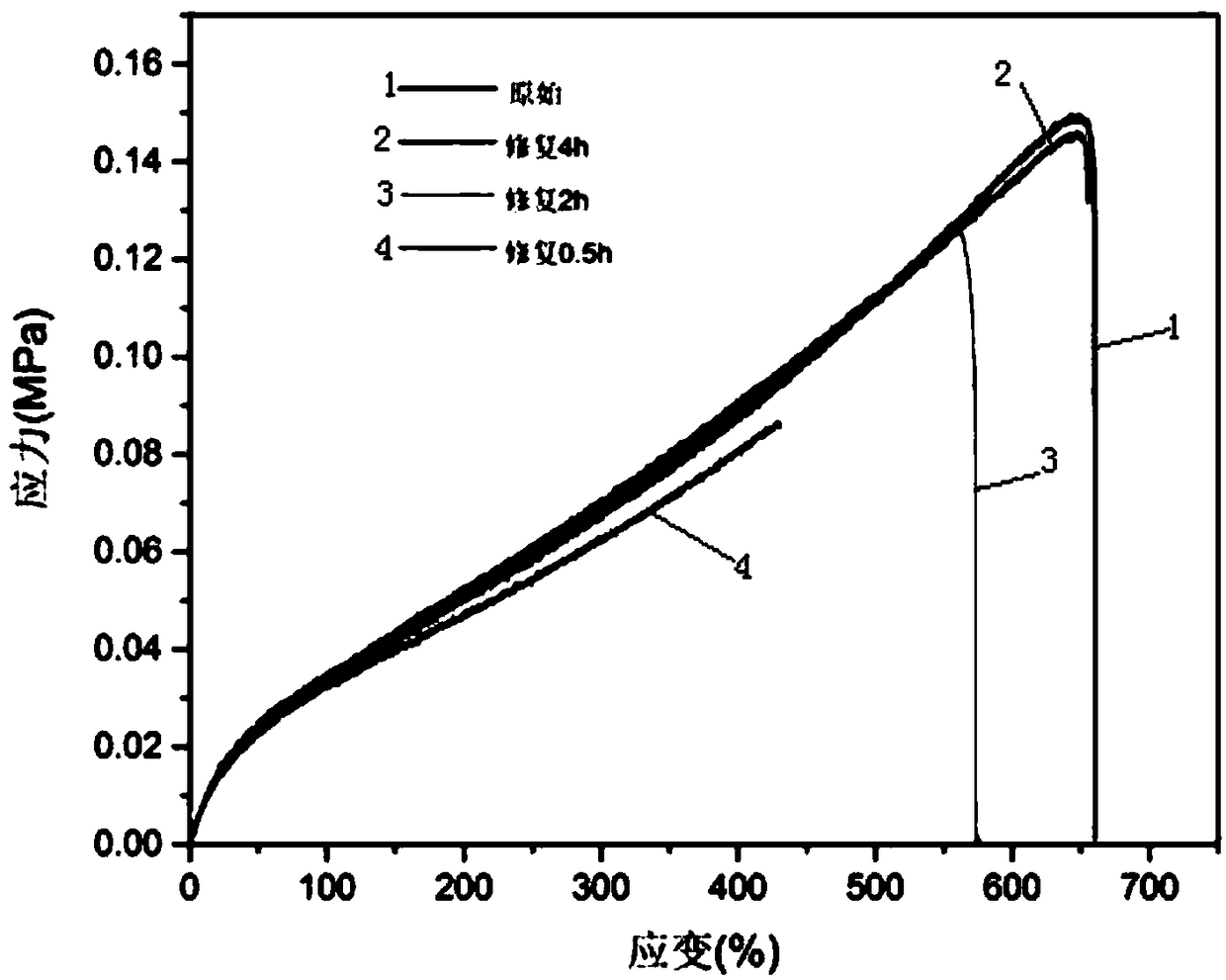
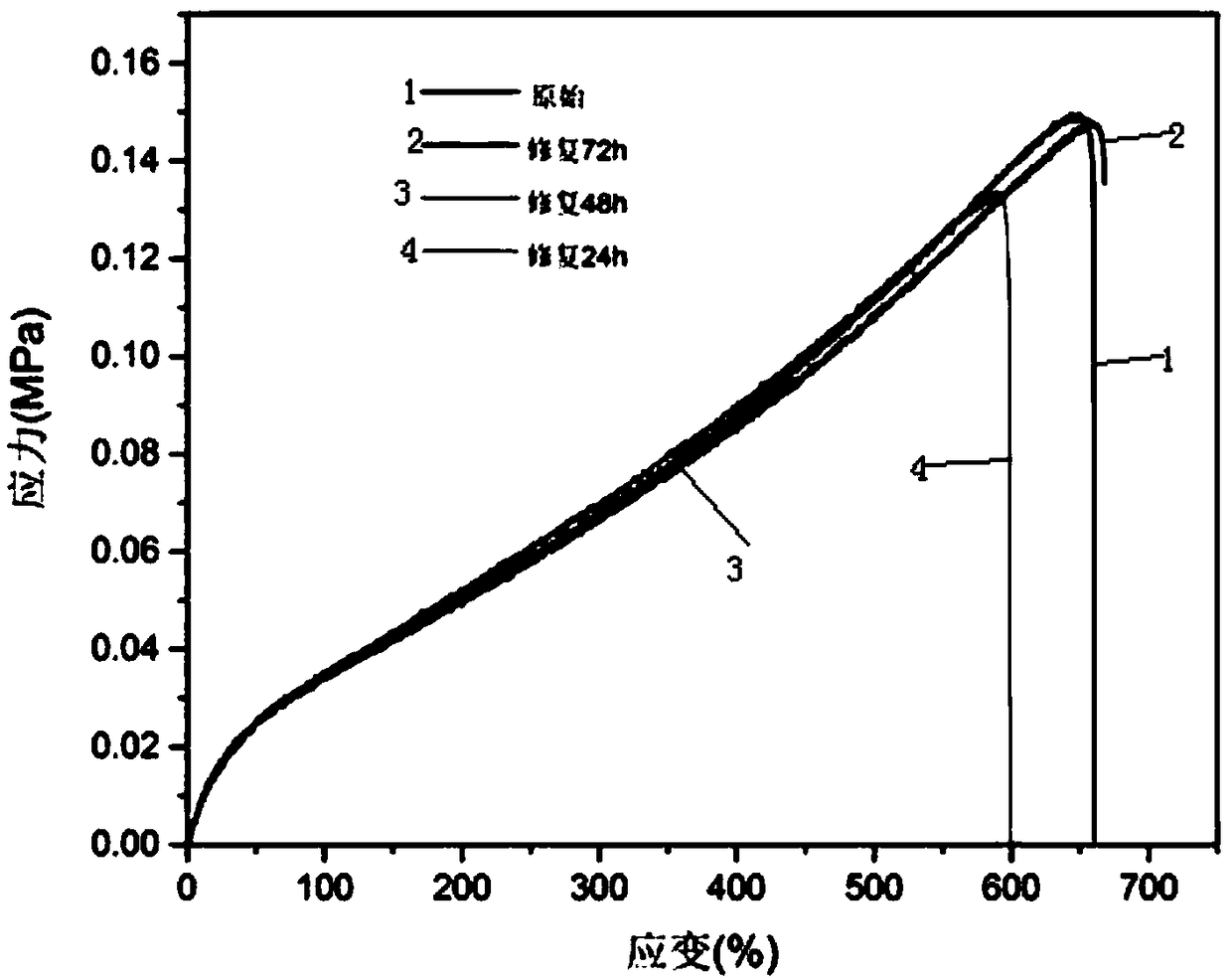
Application of polysiloxane elastomer based on aryl disulfide bond and imine bond as self-healing material
A technology of polysiloxane and healing materials, which is applied in the field of polysiloxane elastomer and its preparation, can solve the problems of harsh conditions, difficult implementation, stimulation, etc., and achieve the effect of low cost, simple synthesis process and significant application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Evacuate the 50 mL reaction flask, and perform an air extraction-inflation process three times to eliminate oxygen in the system. Put 5 g of polydimethylsiloxane and 0.248 g of 4,4'-diaminodiphenyl disulfide in the reaction flask, and then fill and deflate once more. Add 20 mL of anhydrous dichloromethane by injection, stir well to make it completely dissolved.
[0032] (2) Evacuate the 50 mL reaction flask, and perform an air extraction-inflation process three times to remove oxygen in the system. Put 0.162g of 1,3,5-trityl and 0.015g of zinc aldehyde trifluoromethanesulfonate in the reaction flask, and then fill and deflate once more. Add 10 mL of anhydrous dichloromethane by injection, stir well to make it completely dissolved.
[0033] (3) Use a needle to extract the solution in step (1) and add it to the solution in step (2), react at room temperature for 24 hours, pour the reaction solution into a tetrafluoroethylene mold, and place the mold in a fume hood to vol...
Embodiment 2
[0035] (1) Evacuate the 50 mL reaction flask, and perform an air extraction-inflation process three times to eliminate oxygen in the system. Put 5g of polydimethylsiloxane and 0.062g of 4,4'-diaminodiphenyl disulfide in the reaction flask, and fill and deflate once more. Add 20 mL of anhydrous dichloromethane by injection, stir well to make it completely dissolved.
[0036] (2) Evacuate the 50 mL reaction flask, and perform an air extraction-inflation process three times to remove oxygen in the system. Put 0.081g of 1,3,5- mesitylene and 0.011g of zinc aldehyde trifluoromethanesulfonate in the reaction flask, and then fill and deflate once more. Add 10 mL of anhydrous dichloromethane by injection, stir well to make it completely dissolved.
[0037] (3) Use a needle to extract the solution in step (1) and add it to the solution in step (2), react at room temperature for 24 hours, pour the reaction solution into a tetrafluoroethylene mold, and place the mold in a fume hood to volat...
Embodiment 3
[0039] (1) Evacuate the 50 mL reaction flask, and perform an air extraction-inflation process three times to eliminate oxygen in the system. Put 5 g of polydimethylsiloxane and 0.248 g of 4,4'-diaminodiphenyl disulfide in the reaction flask, and then fill and deflate once more. Add 20 mL of anhydrous dichloromethane by injection, stir well to make it completely dissolved.
[0040] (2) Evacuate the 50 mL reaction flask, and perform an air extraction-inflation process three times to remove oxygen in the system. Put 0.162g of 1,3,5-trityl and 0.025g of Europium aldehyde trifluoromethanesulfonate in the reaction flask, and then fill and deflate once more. Add 10 mL of anhydrous dichloromethane by injection, stir well to make it completely dissolved.
[0041] (3) Use a needle to extract the solution in step (1) and add it to the solution in step (2), react at room temperature for 1 hour, pour the reaction solution into a tetrafluoroethylene mold, and place the mold in a fume hood to v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com