Chimeric antigen receptor-modified immune effector cell co-expressing PD-L1 blocker
An immune effector cell, chimeric antigen receptor technology, applied in genetically modified cells, cells modified by introducing foreign genetic material, blood/immune system cells, etc., can solve the problem of poor survival rate and low activity of immune effector cells , the effect is not obvious, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100]Example 1. Construction of recombinant lentiviral vector PRRLSIN-cPPT.EF-1α-sPD1-CH3-F2A-9F2-CAR in which soluble sPD1-CH3 binds to a chimeric antigen (GPC3) receptor
[0101] 1. Design human sPD1, sPD1-CH3 and sPD1-Fc sequences
[0102] 1) Human sPD1 sequence design
[0103] The PD-1 DNA sequence refers to the pubmed Nucleotide database, and the corresponding number of PD-1 is NM_005018.2; the signal peptide and extracellular segment of PD-1 refer to the Uniprot database.
[0104] PD-1 signal peptide sequence:
[0105] ATGCAGATCCCACAGGCGCCCTGGCCAGTCGTCTGGGCGGTGCTACAACTGGGCTGGCGG (SEQ ID NO: 1).
[0106] PD-1 extracellular segment sequence:
[0107] CCAGGATGGTTCTTAGACTCCCCAGACAGGCCCTGGAACCCCCCCACCTTCTCCCCAGCCCTGCTCGTGGTGACCGAAGGGGACAACGCCACCTTCACCTGCAGCTTCTCCAACACATCGGAGAGCTTCGTGCTAAACTGGTACCGCATGAGCCCCAGCAACCAGACGGACAAGCTGGCCGCCTTCCCCGAGGACCGCAGCCAGCCCGGCCAGGACTGCCGCTTCCGTGTCACACAACTGCCCAACGGGCGTGACTTCCACATGAGCGTGGTCAGGGCCCGGCGCAATGACAGCGGCACCTACCTCTGTGGGGCCATCTCCCTG...
Embodiment 2
[0132] Example 2. Lentivirus preparation and expression of T lymphocyte chimeric antigen receptor
[0133] 1. Lentiviral packaging
[0134] (1) 293T cell inoculation: according to 6×10 6 Inoculate the 293T cells cultured to the 6th to 10th passages in a 10cm culture dish at a density of 37°C and 5% CO 2 Cultivate overnight to prepare for transfection, the medium is antibiotic-free DMEM containing 10% fetal bovine serum (Gibco), and the next day, prepare for transfection when the cell abundance is about 70%-80%.
[0135] (2) Transfection:
[0136] Plasmid to be transfected: plasmid pRRL-EF-1α-AB1-28Z (plasmid 1) or plasmid expressing sPD-1-CH3 protein targeting GPC3 chimeric antigen receptor (plasmid 2)
[0137] Use PEI for transfection, use four-plasmid lentivirus packaging system, prepare PEI / DNA mixture A preparation: take 5.4 μg of plasmid 1 or plasmid 2 (plasmid to be transfected), 6.2 μg of pMDLg-pRRE, 6.2 μg of pRSV-Rev, pCMV-VSV-G2.4 μg. Dissolve the plasmid in 800...
Embodiment 3
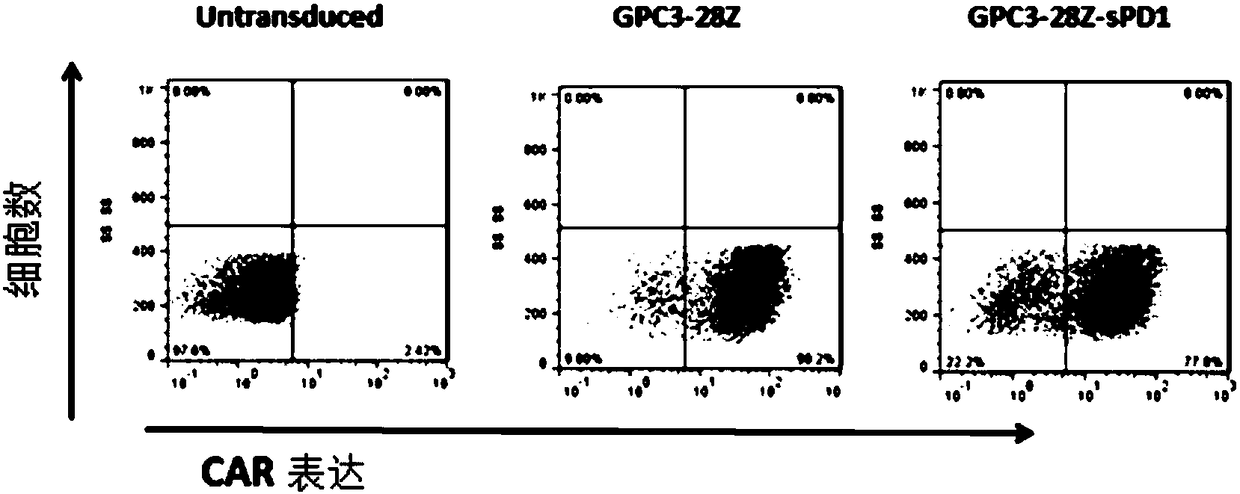
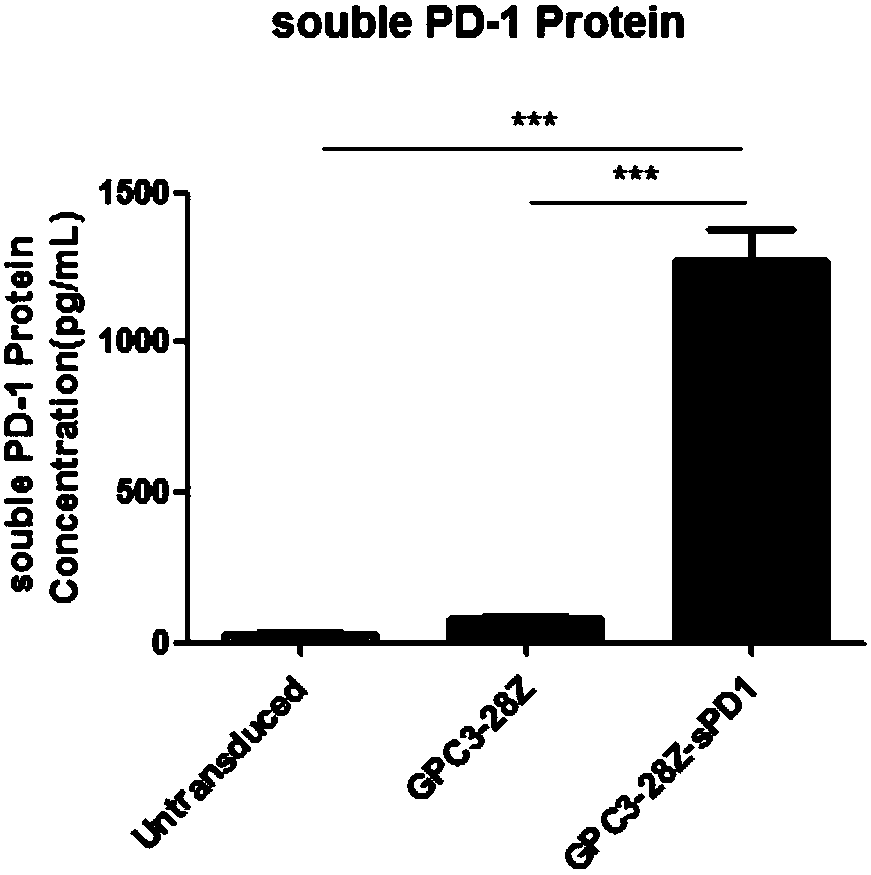
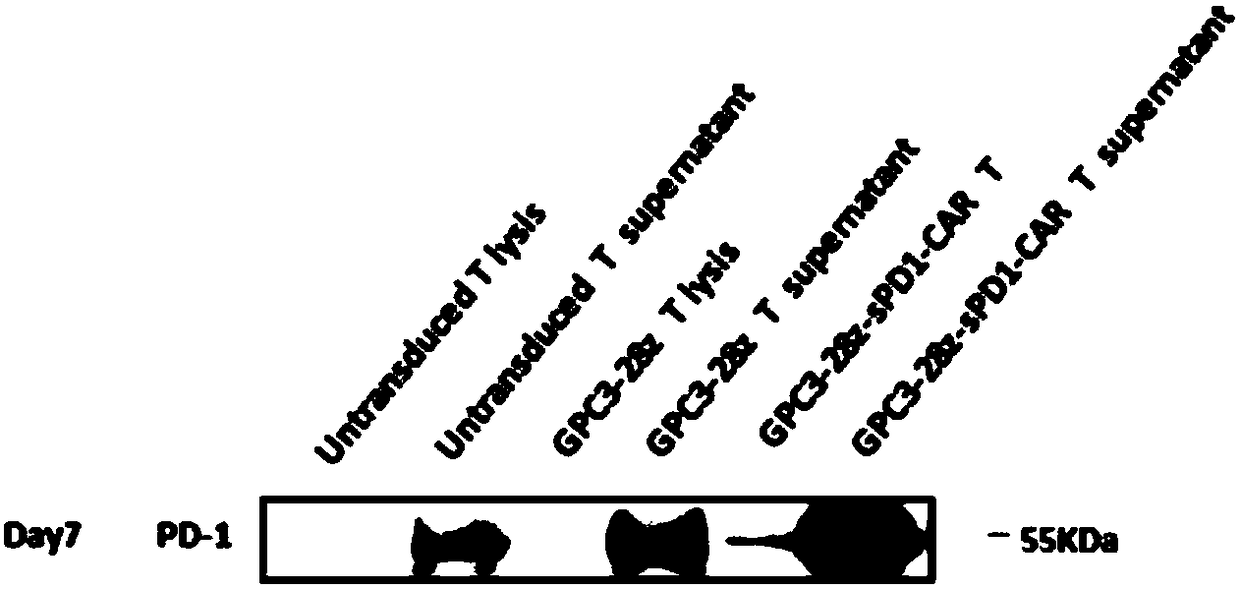
[0148] Example 3. Expression of Senescence Markers on the Surface of CAR-T Cell Membranes
[0149] GPC3-positive SK-HEP-1-GPC3 was selected as target cells, and the target cells were cultured with CAR-T cells co-expressing sPD1 (GPC3-28Z-sPD1) and CAR-T cells not expressing sPD1 on day 11 in vitro. (GPC3-28Z) were co-incubated at a ratio of 1:1, and the target cells were stimulated once every 24 hours for a total of 72 hours, and 5×10 5 The T cells in the EP tube were washed twice with the ice-bathed flow washing solution (prepared with 1% NCS plus PBS), diluted 1:50 and added to Anti-human CD279(PD-1) (eBioscience PerCP-eFluor○ R CLONE: eBioJ105); Anti-human CD366 (TIM-3) (eBioscience APC CLONE: F38-2E2); Anti-human CD223 (Lag-3) (eBioscience eFluor○R450 CLONE: 3DS223H) direct labeled antibody, incubated on ice After 45min, vortex washing for three times, T cell exhaustion markers PD-1, TIM-3, and Lag-3 were detected by flow cytometry, and the results were as follows: imag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com