Method for separating and analyzing optical isomers of bedaquiline
A technology of optical isomers and bedaquiline, applied in the field of liquid chromatography, can solve problems such as difficult quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Instruments and Conditions
[0035] Chromatographic column: Daicel CHIRALPAKZWIX (+) (4.6*150mm, 3μm);
[0036] Mobile phase: 2.6mL diethylamine, 1.9mL formic acid to 1000mL methanol;
[0037] Column temperature: 12°C;
[0038] Wavelength: 226nm;
[0039] Flow rate: 0.1mL / min;
[0040] Injection volume: 1 μL.
[0041] Experimental procedure
[0042] (1S,2R)-bedaquiline stock solution: Weigh 10mg of (1S,2R)-bedaquiline, put it in a 10mL measuring bottle, dilute to the mark with methanol, shake well, then pipette 1mL and put it in a 100mL volume bottle, dilute to volume with methanol.
[0043] Test solution: Weigh 10mg of (1R,2S)-bedaquiline, weigh it accurately, put it in a 20mL measuring bottle, add methanol to dissolve and dilute to the mark, and shake well.
[0044] System suitability solution: Weigh 10mg of (1R,2S)-bedaquiline into a 10mL volumetric flask, pipette 1.0mL of (1S,2R)-bedaquiline stock solution into this volumetric flask, add methanol to dissolve ...
Embodiment 2
[0050] Instruments and Conditions
[0051] Chromatographic column: Daicel CHIRALPAKZWIX (+) (4.6*150mm, 3μm);
[0052] Mobile phase: 2.6mL diethylamine, 1.9mL formic acid to 1000mL methanol;
[0053] Column temperature: 12°C;
[0054] Wavelength: 226nm;
[0055] Flow rate: 0.08mL / min;
[0056] Injection volume: 1 μL.
[0057] The experimental procedure is the same as in Example 1.
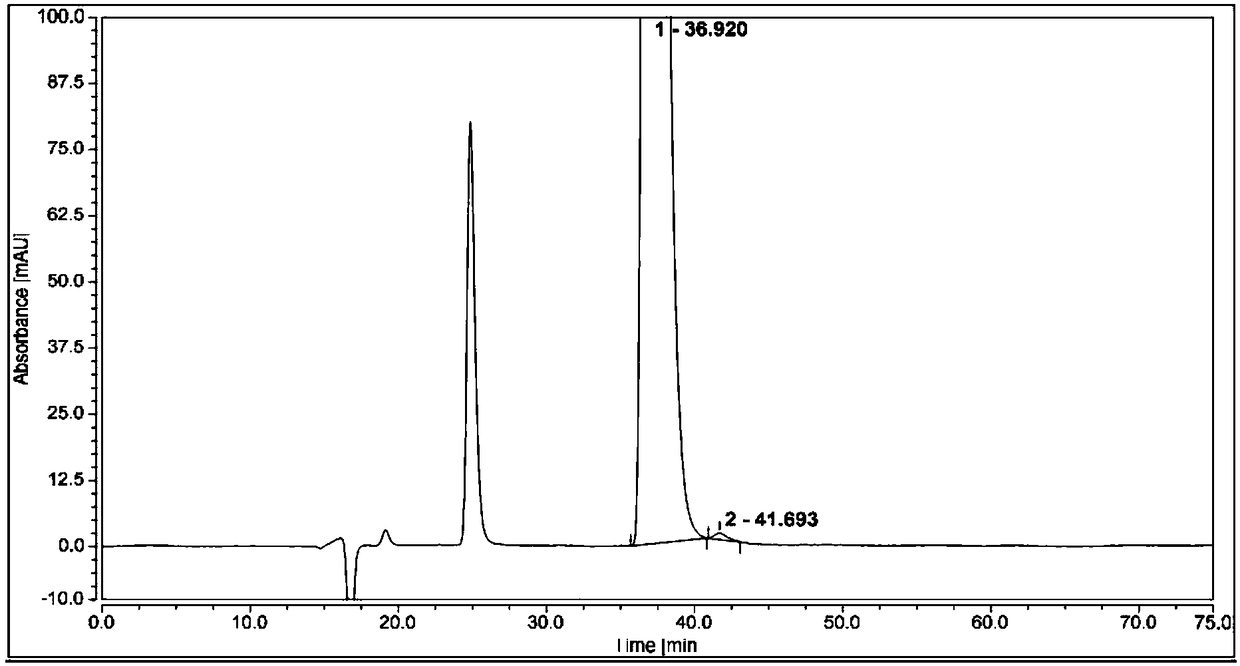
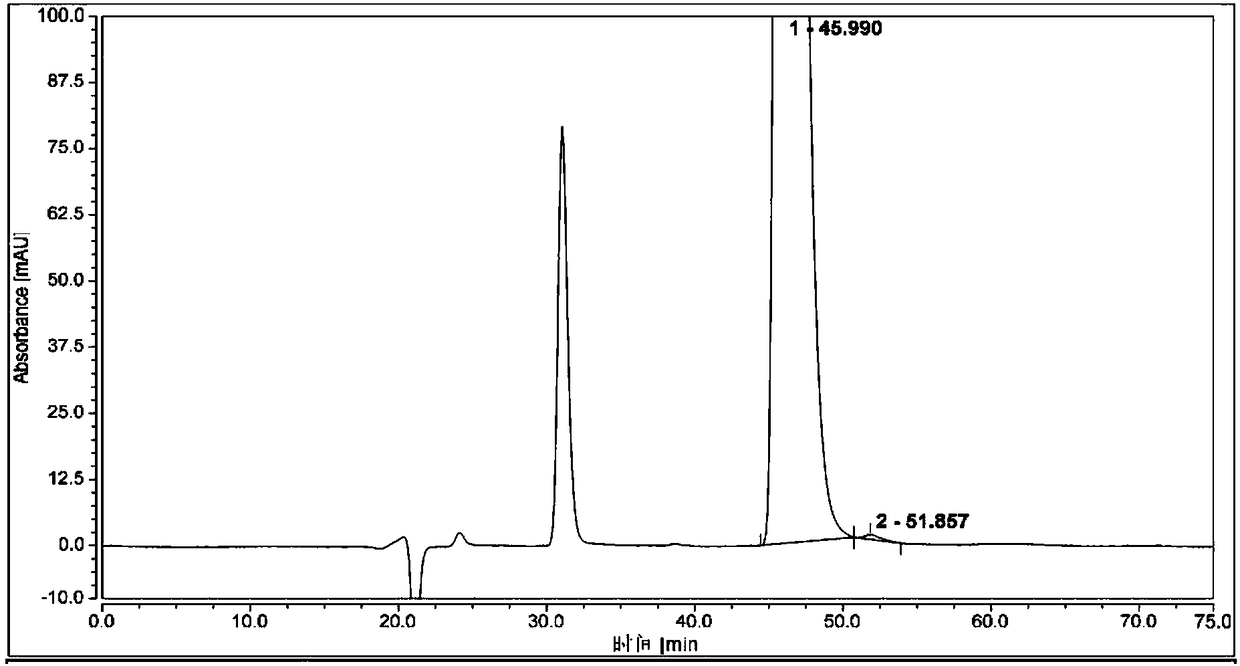
[0058] Measure 1 μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram. The resolution of (1S,2R)-bedaquiline peak and (1R,2S)-bedaquiline peak is greater than 1.5.
[0059]Take 1 μL of the test solution and (1R,2S)-bedaquiline self-contrast solution respectively, perform high-performance liquid chromatography analysis according to the above conditions, and record the chromatograms. The content of (1S, 2R)-bedaquiline was calculated by adding a correction factor to the self-control method, and the content was 0.2%.
[0060] see attached r...
Embodiment 3
[0062] Instruments and Conditions
[0063] Chromatographic column: Daicel CHIRALPAKZWIX (+) (4.6*150mm, 3μm);
[0064] Mobile phase: 2.6mL diethylamine, 1.9mL formic acid to 1000mL methanol;
[0065] Column temperature: 12°C;
[0066] Wavelength: 226nm;
[0067] Flow rate: 0.09mL / min;
[0068] Injection volume: 1 μL.
[0069] The experimental procedure is the same as in Example 1.
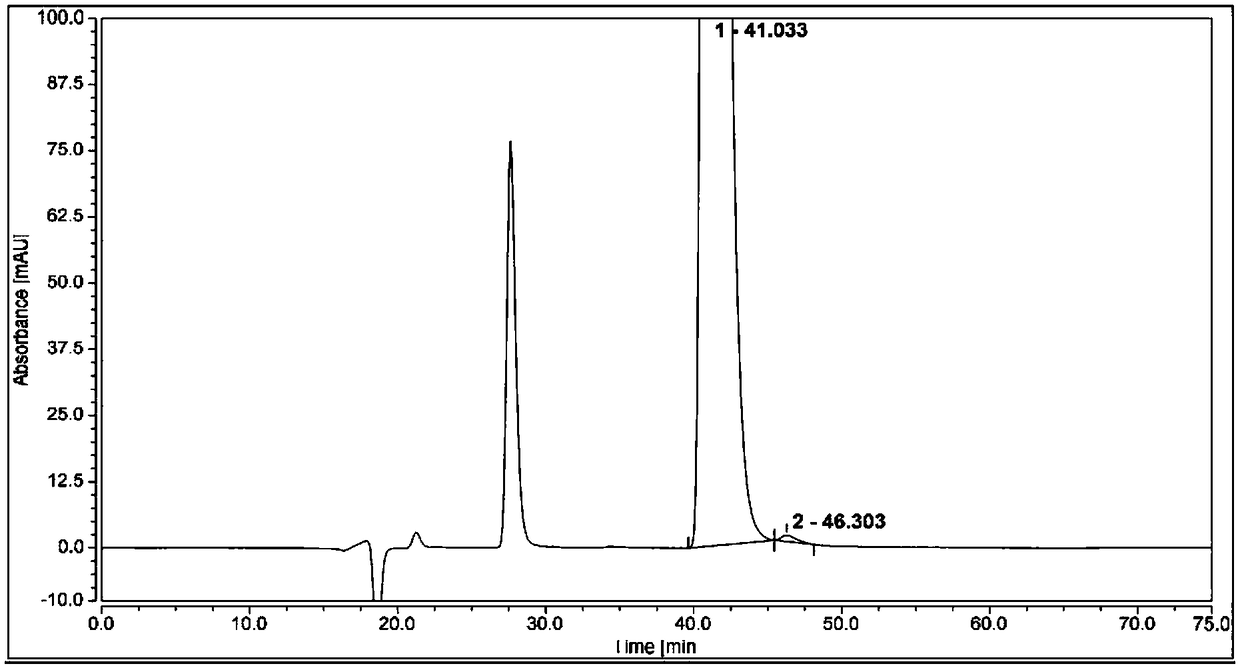
[0070] Measure 1 μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram. The resolution of (1S,2R)-bedaquiline peak and (1R,2S)-bedaquiline peak is greater than 1.5.
[0071] Take 1 μL of the test solution and (1R,2S)-bedaquiline self-contrast solution respectively, perform high-performance liquid chromatography analysis according to the above conditions, and record the chromatograms. The content of (1S, 2R)-bedaquiline was calculated by adding a correction factor to the self-control method, and the content was 0.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com