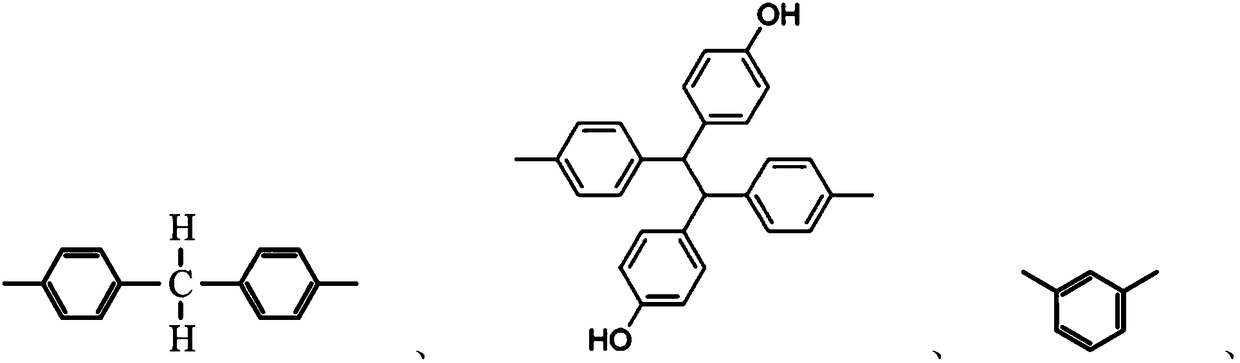
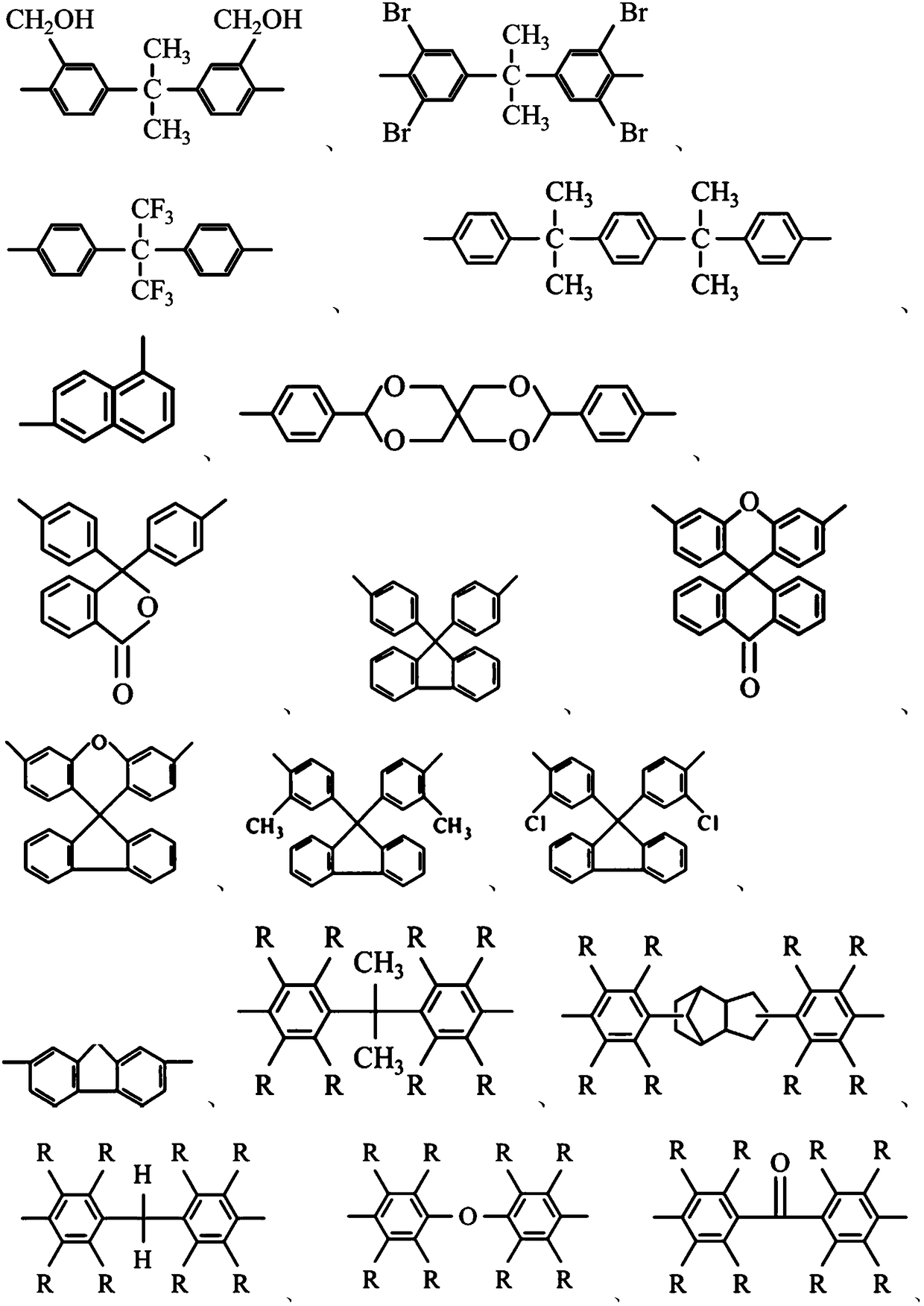
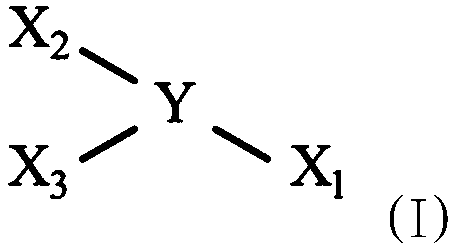Reactive epoxy compounds and method for producing the same, core-shell type epoxy resin particles, waterborne epoxy resin composition, and coating composition containing the reactive epoxy compounds
A technology of water-based epoxy resin and epoxy resin particles, which is used in the fields of low-volatile organic compounds, water-based epoxy resin compositions, and reactive surfactants, and can solve the problems of low mechanical stability, poor adhesion, and epoxy resin emulsions. Poor water resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1-synthesis of this reactive epoxy compound (S1)
[0053] 9.6g trimellitic anhydride (TMA) and 200g polyether amine (purchased from HUNTSMAN company, trade name M-2070) were mixed and reacted with 0.04 g of triphenylphosphine (triphenylphosphine, TPP) as a catalyst at 120°C. 18.8g of bisphenol-A epoxy resin (purchased from CCP Company, trade name BE188) and 0.05g of TPP were further added into the reaction, and reacted at 140°C. The final product has the S1 structure as described above with an acid value of 0.77.
Embodiment 2
[0054] Embodiment 2-synthesis of this reactive epoxy compound (S2)
[0055] 24g trimellitic anhydride (TMA) and 250g polyether amine (purchased from HUNTSMAN company, trade name M-2070) were mixed and reacted with 0.05 g of triphenylphosphine (TPP) as a catalyst at 120°C. 94g of bisphenol-A epoxy resin (purchased from CCP Company, trade name BE188) and 0.07g of TPP were further added into the reaction, and reacted at 140°C. The final product has the S2 structure as described above with an acid value of 0.68.
Embodiment 3
[0056] Embodiment 3-synthesis of this reactive epoxy compound (S3)
[0057] 9.6 g of trimellitic anhydride (TMA) and 93.8 g of PEG1500-BE188 were reacted at 120° C. with 0.02 g of triphenylphosphine (TPP) as catalyst. With 100g polyether amine (purchased from HUNTSMAN company, trade name M-2070) was further added to the reaction, and reacted with 0.04 g of triphenylphosphine (TPP) as a catalyst at 120°C. Finally, 18.8g of bisphenol-A epoxy resin (purchased from CCP Company, trade name BE188) and 0.04g of TPP were further added into the reaction, and reacted at 140°C. The final product has the S3 structure as described above and an acid value of 0.94.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com