Lactobacillus gasseri and application thereof in preparing premature delivery preventing medicine
A technology of Lactobacillus gasseri and medicines, applied in the field of medical microorganisms, can solve problems such as reducing the premature birth rate and developing drug resistance, and achieve the effects of ensuring the number of viable bacteria, inhibiting Gardnerella, and reducing the risk of premature birth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This implementation is used to illustrate the antagonistic effect of the bacterial strain of the present invention on Gardnerella vaginalis. The Gardnerella vaginalis used in this example is the strain purchased from ATCC with the product number 10231.
[0047] Cultivate Lactobacillus gasseri H87 with MRS liquid medium to the concentration of bacteria
[0048] 1×10 7 CFU / mL culture medium, named as culture medium A.
[0049] Gardnerella vaginalis was cultured with MRS liquid medium to a cell concentration of 1×10 7 CFU / mL culture medium, named as culture medium B.
[0050] The above-mentioned culture solution A and the above-mentioned culture solution B were mixed in equal volumes as an experimental group, and the above-mentioned culture solution B alone was used as a control group.
[0051] 1 mL of the culture liquid of the experimental group and the control group were respectively inoculated into 150 mL of the MRS liquid medium, and cultured at 37° C. for 24 hours...
Embodiment 2
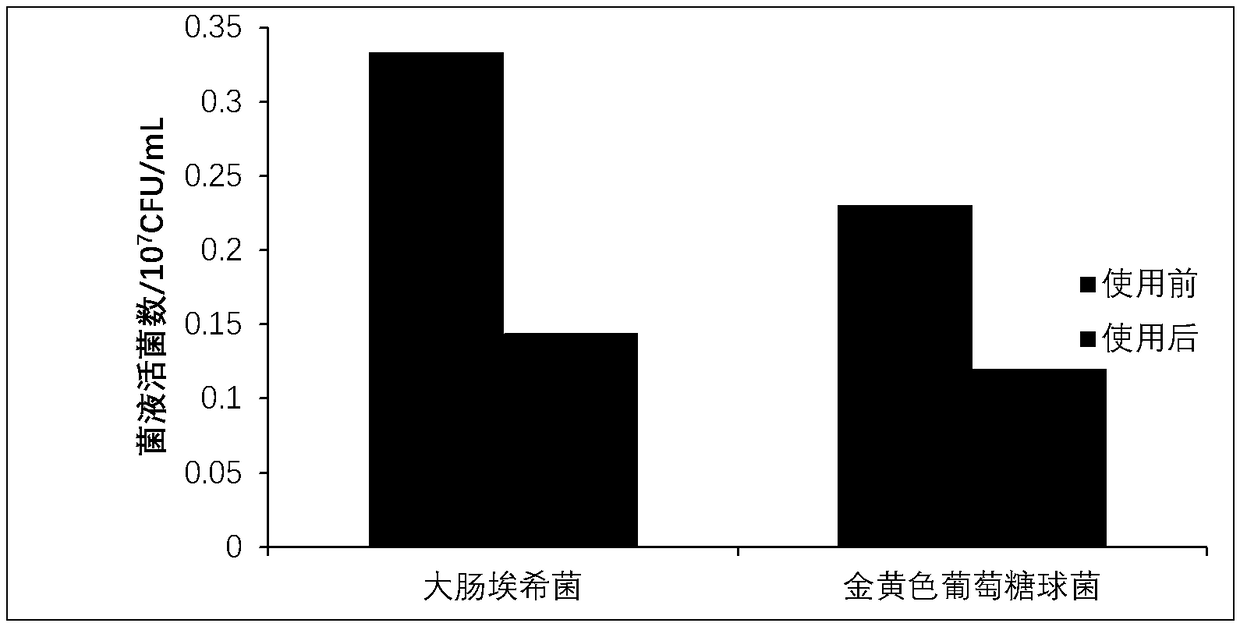
[0058] This example is used to illustrate the antagonistic effect of the strain of the present invention on Escherichia coli. The Escherichia coli used in this example is the strain purchased from ATCC with the product number 35218.
[0059] Lactobacillus gasseri H87 was cultured with MRS liquid medium to a concentration of 1×10 7 CFU / mL culture medium, named as culture medium A.
[0060] Escherichia coli was cultured with MRS liquid medium to a concentration of 1×10 7 CFU / mL culture medium, named as culture medium B.
[0061] The above-mentioned culture solution A and the above-mentioned culture solution B were mixed in equal volumes as an experimental group, and the above-mentioned culture solution B alone was used as a control group.
[0062] 1 mL of the culture liquid of the experimental group and the control group were respectively inoculated into 150 mL of the MRS liquid medium, and cultured at 37° C. for 24 hours to obtain a co-cultivation culture liquid.
[0063] T...
Embodiment 3
[0069] This example is used to illustrate the antagonistic effect of the strains of the present invention on Staphylococcus aureus. The Staphylococcus aureus used in this example is the strain purchased from ATCC with the product number 35218.
[0070] Lactobacillus gasseri H87 was cultured with MRS liquid medium to a concentration of 1×10 7 CFU / mL culture medium, named as culture medium A.
[0071] Staphylococcus aureus was cultured with MRS liquid medium to a concentration of 1×10 7 CFU / mL culture medium, named as culture medium B.
[0072] The above-mentioned culture solution A and the above-mentioned culture solution B were mixed in equal volumes as an experimental group, and the above-mentioned culture solution B alone was used as a control group.
[0073] 1 mL of the culture liquid of the experimental group and the control group were respectively inoculated into 150 mL of the MRS liquid medium, and cultured at 37° C. for 24 hours to obtain a co-cultivation culture liq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com