Method for producing cd4/cd8 double-positive t cells
A double-positive, pluripotent stem cell technology, applied in the field of preparation of CD4CD8 double-positive T cells, can solve the problems of difficult differentiation regulation, low self-regeneration ability, low gene transfer efficiency, etc., and achieve the effect of efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] In the production of CD4CD8 double-positive T cells, hematopoietic progenitor cells can be cultured using feeder cells, but preferably cultured without using feeder cells.
[0081] Hematopoietic progenitor cells can be cultured adherently or in suspension, but adherent culture is preferred in this step. In the case of adherent culture, the culture container can be coated and used. For example, as a coating agent, Matrigel can be cited (Niwa A, et al.PLoS One.6(7):e22261, 2011) , collagen, gelatin, laminin, heparan sulfate proteoglycan, RetroNectin, Fc-DLL4 or nestin, and combinations thereof.
[0082] In addition, when hematopoietic progenitor cells are obtained by suspension culture of embryoid bodies, it is preferable to perform adherent culture after dissociation into single cells.
[0083] In the present invention, the culture temperature conditions for culturing hematopoietic progenitor cells for the preparation of CD4CD8 double-positive T cells are not pa...
Embodiment 1
[0132] On ultra-low adhesion treated 6-well plates (CORNING: #3471), 3x10 5 ~6x10 5 Each well was inoculated with TkT3V1-7, FfI-01, Ffl-14 or KhES-3 (day 0), and added 10 μg / ml human insulin, 5.5 μg / ml human transferrin, 5ng / ml sodium selenite, 2mM L-glutamine, 45mMα-monothioglycerol, 50μg / ml ascorbic acid) added 10ng / ml BMP4, 5ng / ml bFGF, 15ng / ml VEGF, 2μM SB431542 and in hypoxia Under the condition (5%O 2 ) were cultured for 5 days (Day 5).
[0133] Next, 50 ng / ml of SCF, 30 ng / ml of TPO, and 10 ng / ml of Flt3L were added, and further culture was carried out for 5 to 9 days (to day 14).
[0134] The obtained hematopoietic progenitor cells were plated on a 48-well plate coated with Fc-DLL4 (5 μg / ml) (Sino Biological Inc.) and Retronectin (5 μg / ml) (Takara Bio Co., Ltd.), and added with 50 ng / ml of SCF, OP9 medium (15% FBS, 2mM L-glutamine, 100Uml penicillin , 100ng / ml streptomycin, 55μM 2-mercaptoethanol, 50μg / ml ascorbic acid, 10μg / ml human insulin, 5.5μg / ml hum...
Embodiment 2
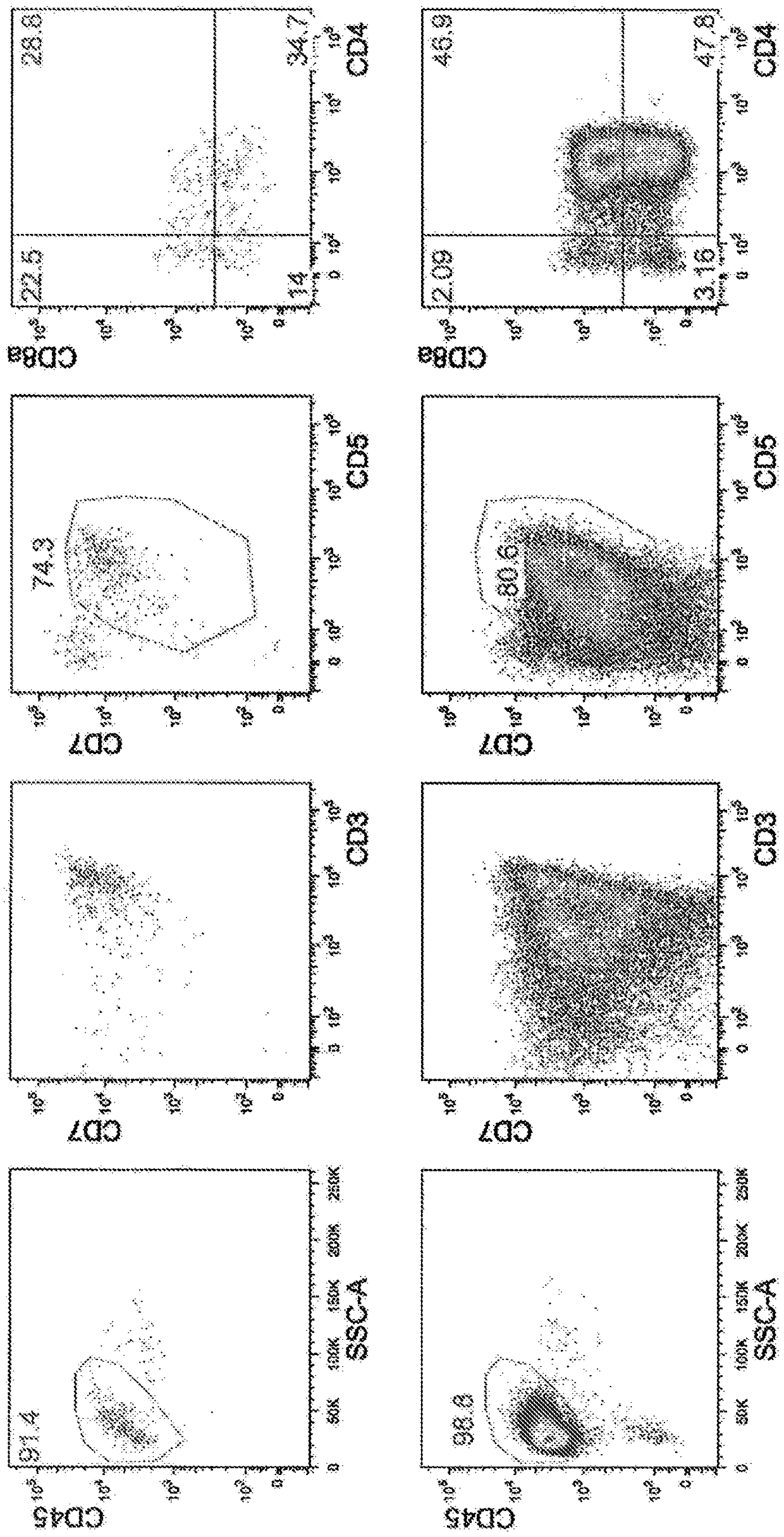
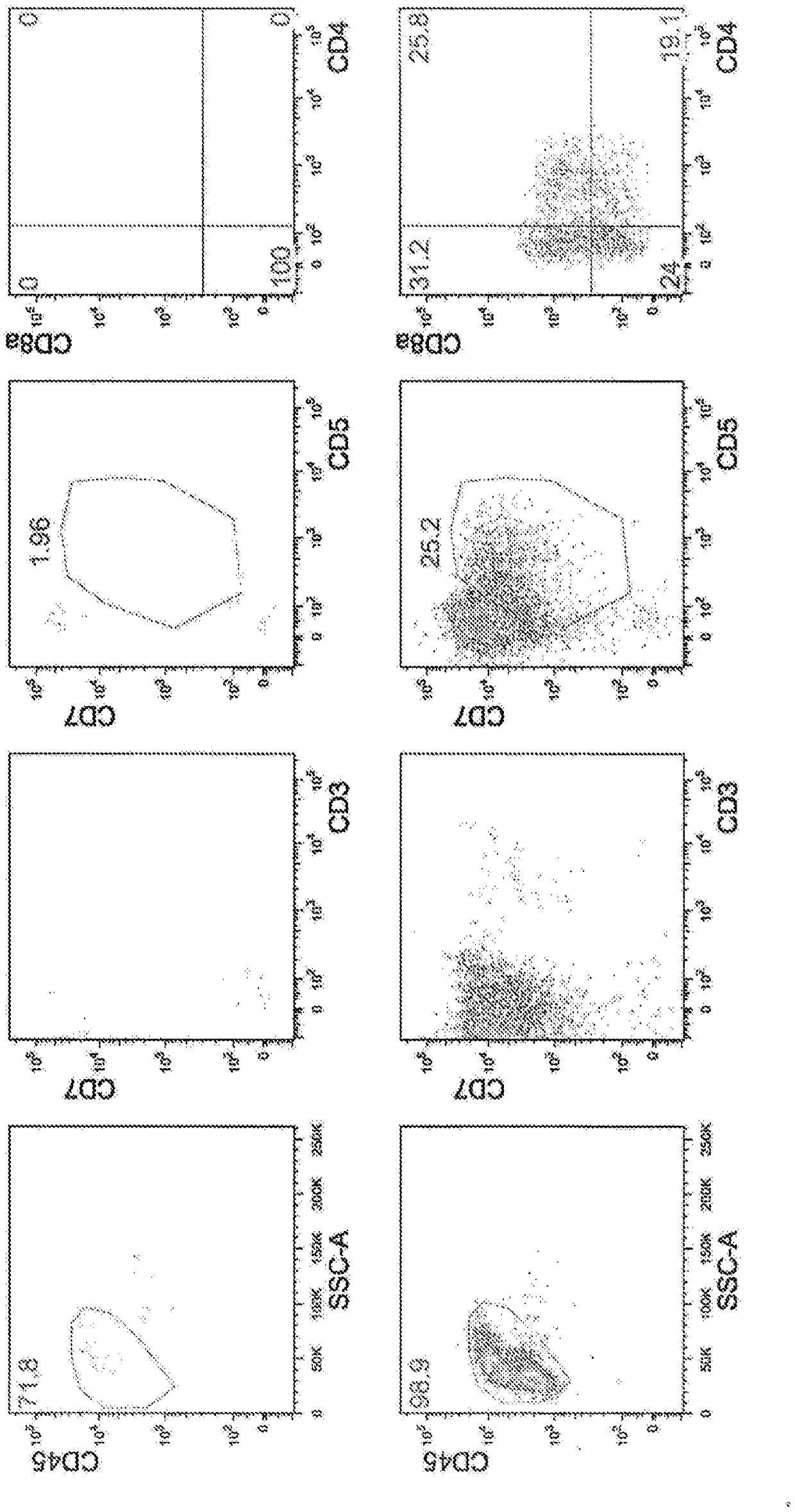
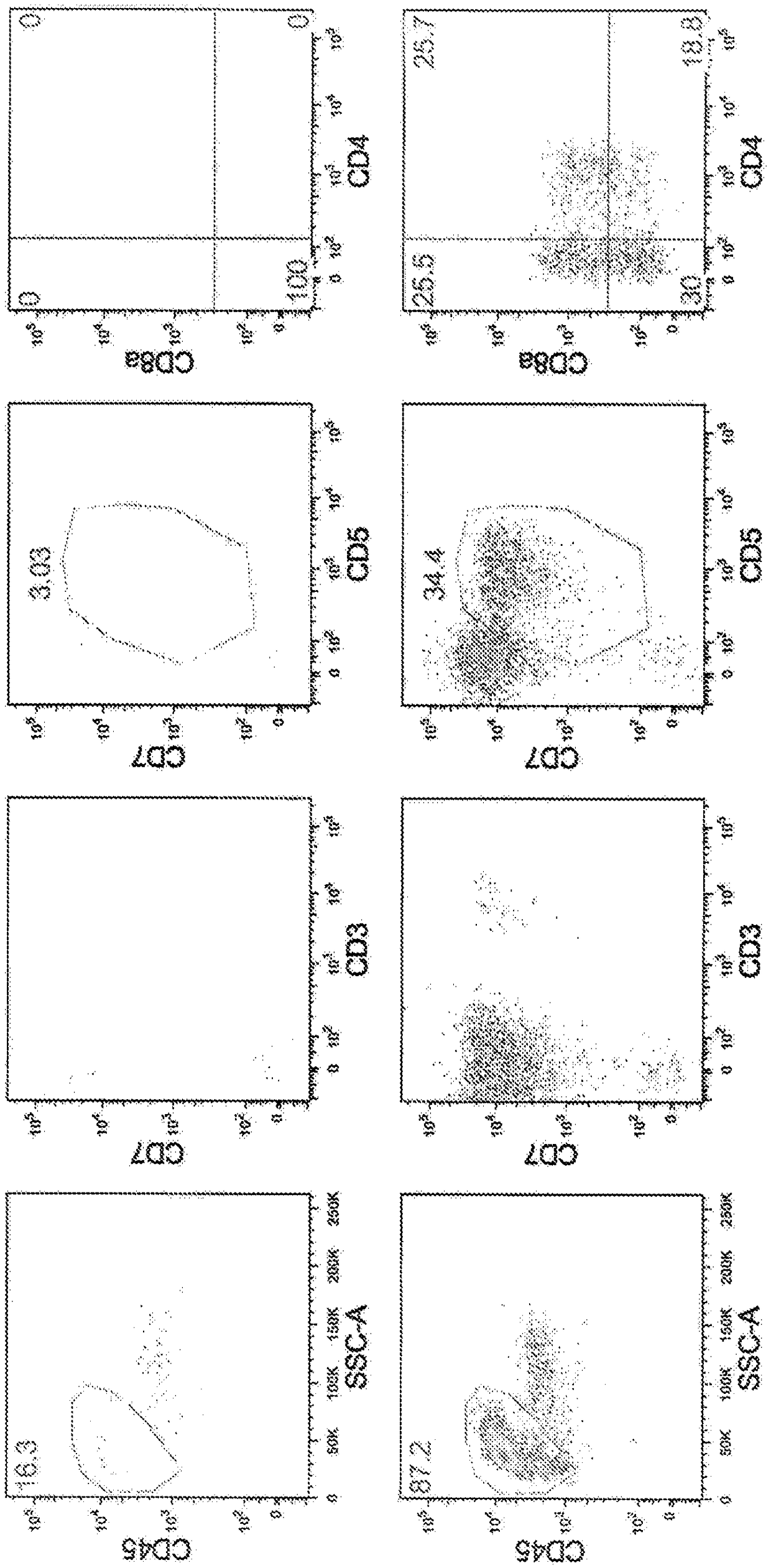
[0139] CD34-positive hematopoietic progenitor cells from umbilical cord blood (HemaCare #CBCD34C-1) were coated with Fc-DLL4 (5 μg / ml) and Retronectin (5 μg / ml) on a 48-well plate, cultured in OP9 medium supplemented with 50ng / ml SCF, 50ng / ml IL-7, 50ng / ml Flt3L, 100ng / ml TPO, 15μMSB203580, and 30ng / ml SDF-1α for 21 days (Day 35). On the 35th day, CD45(+), CD3(+), CD4(+), and CD8(+) components were separated by FACS, and CD4CD8 double positive (Double positive) cells (called DP cells) were obtained. The results are shown in Image 6 . In addition, in Image 6 The upper part of the figure shows the results of culturing hematopoietic progenitor cells without adding SB203580 and SDF-1α. From these results, even when CD34-positive hematopoietic progenitor cells derived from umbilical cord blood were used, CD4CD8 double-positive T cells could be efficiently obtained by culturing in a medium containing a p38 inhibitor and SDF-1.
[0140] In Examples 1 and 2, at the time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com