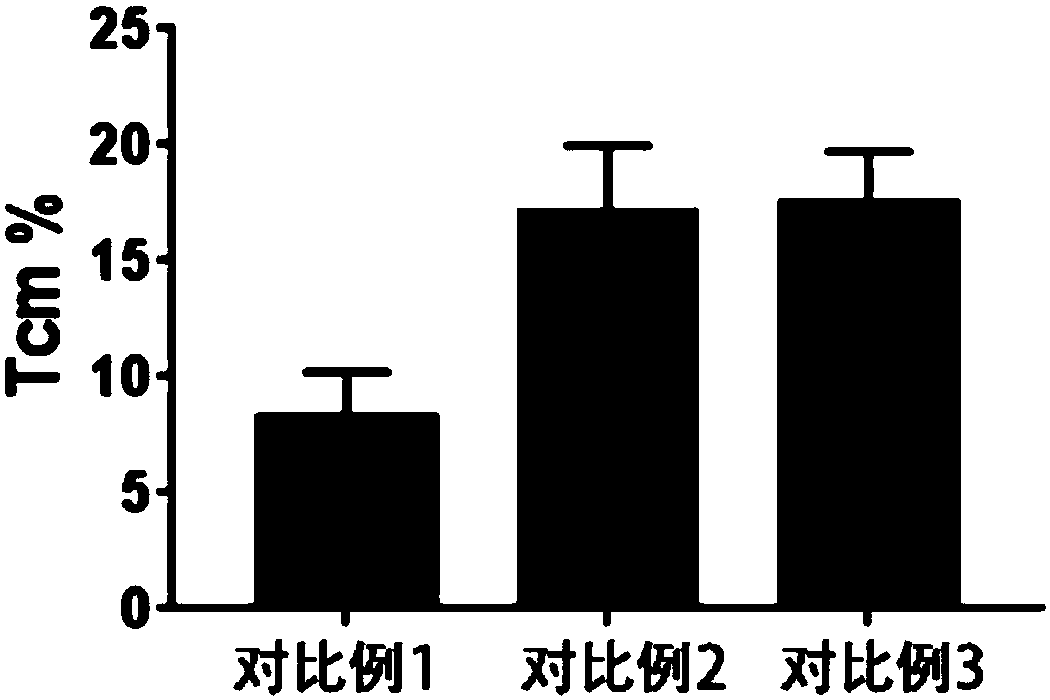
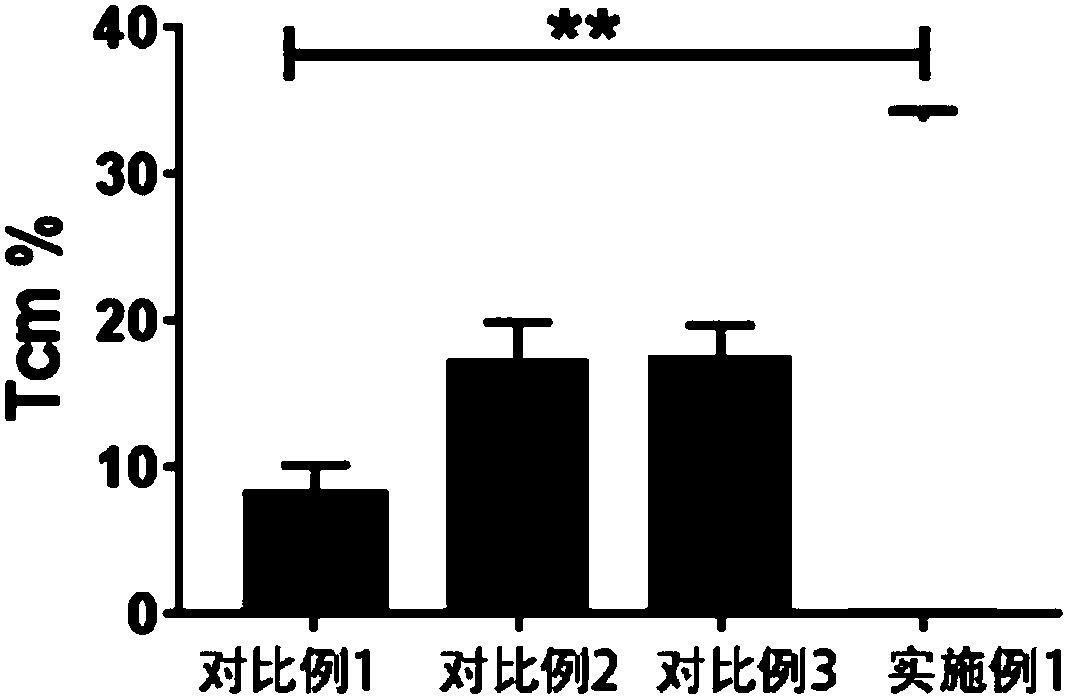
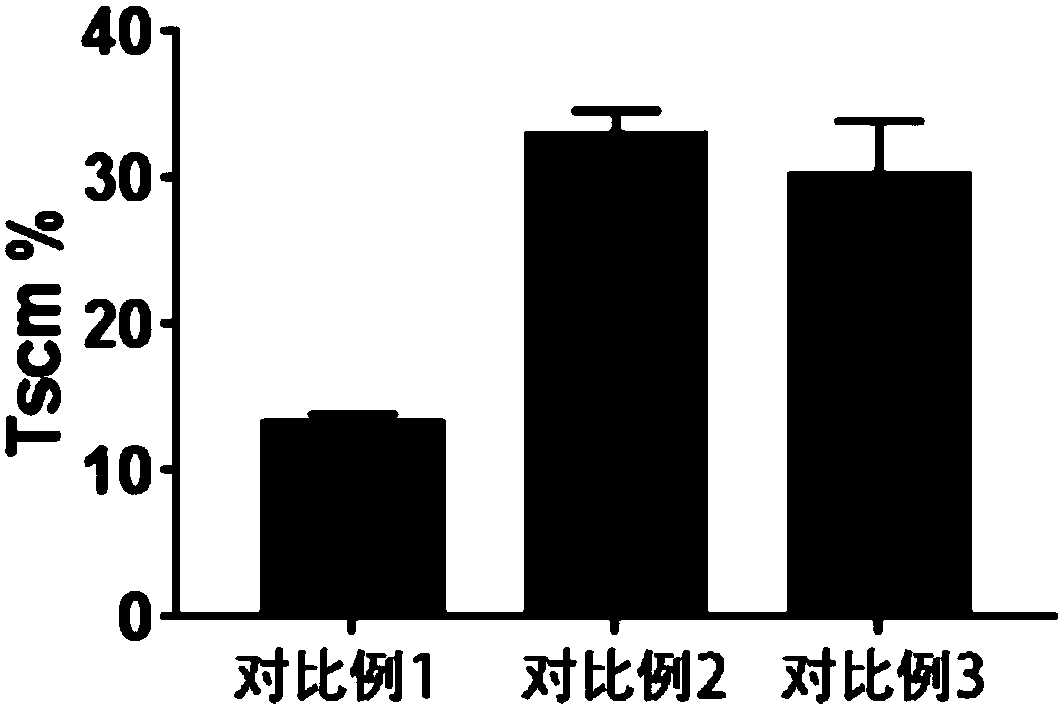
Method for preparing memory T cells
A memory and cellular technology, applied in the field of preparation of human memory T cells, can solve the problems of anti-tumor effect in difficult patients, lack of breakthrough in adoptive cellular immunotherapy, affecting T cell activation and metabolism, etc. Survival time, enhanced anti-tumor ability, enhanced lasting and efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: The preparation method of T cells comprises the following steps
[0033] (1) Collect 20 mL of peripheral blood under sterile conditions and adopt density gradient centrifugation to obtain PBMCs from human peripheral blood mononuclear cells. Both acceleration and deceleration are 9m / s 2 , to obtain serum; B. Transfer the serum obtained in step A to a sterile 50mL centrifuge tube, inactivate at 56°C for 25min, then centrifuge the serum at 4000rpm for 20min, and the acceleration and deceleration accelerations are both 9m / s 2 , using PBS buffer solution to dilute the precipitated blood cells in a ratio of 1:3 to the volume of blood cells to obtain a blood cell dilution; C, then slowly add the blood cell dilution obtained in step B to the lymph separation liquid, the volume of the lymph separation liquid The amount is 1 / 3 of the volume of the blood cell dilution solution. Under the condition of 2500rpm, centrifuge for 25min, and the acceleration and decelerati...
Embodiment 2
[0037] Embodiment 2: The preparation method of T cells comprises the following steps
[0038] (1) Collect 20 mL of peripheral blood under sterile conditions and adopt density gradient centrifugation to obtain PBMCs from human peripheral blood mononuclear cells. Both acceleration and deceleration are 9m / s 2 , to obtain serum; B. Transfer the serum obtained in step A to a sterile 50mL centrifuge tube, inactivate at 56°C for 25min, then centrifuge the serum at 4000rpm for 20min, and the acceleration and deceleration accelerations are both 9m / s 2 , using PBS buffer solution to dilute the precipitated blood cells in a ratio of 1:2 to the volume of blood cells to obtain a blood cell dilution; C, then slowly add the blood cell dilution obtained in step B to the lymph separation liquid, the volume of the lymph separation liquid The amount is 1 / 4 of the volume of the blood cell dilution solution. Under the condition of 2500rpm, centrifuge for 25min, and the acceleration and decelerati...
Embodiment 3
[0042] Embodiment 3: The preparation method of T cell comprises the following steps
[0043] (1) Collect 20 mL of peripheral blood under sterile conditions and adopt density gradient centrifugation to obtain PBMCs from human peripheral blood mononuclear cells. Both the acceleration and deceleration accelerations are 9m / s 2 , to obtain serum; B. Transfer the serum obtained in step A to a sterile 50mL centrifuge tube, inactivate at 56°C for 25min, then centrifuge the serum at 4000rpm for 20min, and the acceleration and deceleration accelerations are both 9m / s 2 , using PBS buffer solution to dilute the precipitated blood cells in a ratio of 1:4 to the volume of blood cells to obtain a blood cell dilution; C, then slowly add the blood cell dilution obtained in step B to the lymph separation liquid, the volume of the lymph separation liquid The volume is 1 / 2 of the volume of the blood cell dilution solution. Under the condition of 2500rpm, centrifuge for 25min, and the accelerati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com