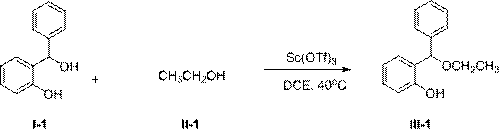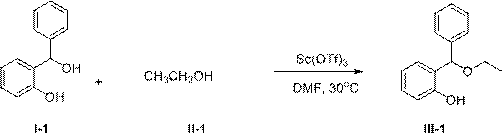Oxa-Michael addition reaction method to generate o-quinone methides in situ by Sc(III) catalysis of alcohols
An in-situ generation technology of methylene quinone, applied in organic chemistry, preparation of ether by dehydration of hydroxyl-containing compounds, preparation of ether, etc., can solve the problems of high price, rare intermolecular oxa-Michael addition reaction, etc., and achieve easy Separation and purification, simple operation and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Add 2.00 g (10 mmol) of compound I-1, 0.92 g (20 mmol) of compound II-1, and 0.49 g (1 mmol) of solid Sc(OTf) into a 100 mL round bottom flask 3 , and finally 50 mL of dry 1,2-dichloroethane was added, and the resulting mixture was stirred at 40 °C for 6 hours. After the reaction mixture was cooled to room temperature, it was poured into ice water, extracted with 50mL×3 methylene chloride, and the extracted organic phases were combined, washed once with saturated brine, anhydrous Na 2 SO 4 Dry, concentrate and remove the solvent to obtain the crude product, which is separated by column chromatography to obtain the pure product of compound III-1. Oily liquid, 2.17g, yield 95%.
Embodiment 2
[0028]
[0029] Add 2.00g (10mmol) compound I-1, 0.92g (20mmol) compound II-1, 0.98g (2mmol) solid Sc(OTf) into a 100mL round bottom flask 3 , and finally 25 mL of dry DMF was added, and the resulting mixture was stirred at 30° C. for 3 hours until the reaction was complete. The reaction mixture was cooled to room temperature, poured into water, stirred, extracted with 50mL×3 dichloromethane, combined and extracted organic phases, washed once with saturated brine, anhydrous Na 2 SO 4 Dry, concentrate and remove the solvent to obtain the crude product, which is separated by column chromatography to obtain the pure product of compound III-1. Oily liquid, 2.23g, yield 98%.
Embodiment 3
[0031]
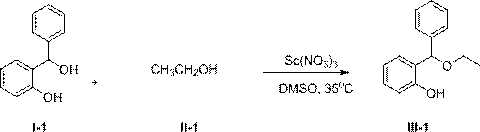
[0032] Add 2.00g (10mmol) compound I-1, 0.69g (15mmol) compound II-1, 0.12g (0.5mmol) solid Sc(NO 3 ) 3 , and finally 70 mL of dry DMSO was added, and the resulting mixture was stirred vigorously at 50 °C for 5 h. After the reaction mixture was cooled to room temperature, it was poured into water, stirred, extracted with 50mL×3 dichloromethane, combined and extracted organic phases were washed once with saturated brine, anhydrous Na 2 SO 4 Dry, concentrate and evaporate the solvent to obtain the crude product, and purify by column chromatography to obtain the pure product of compound III-1. Oily liquid, 2.00g, yield 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com