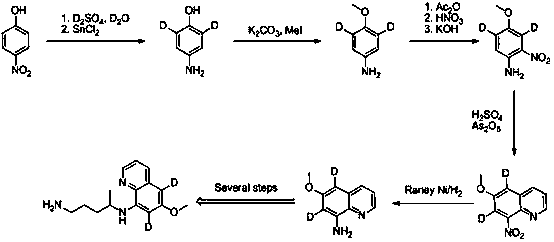
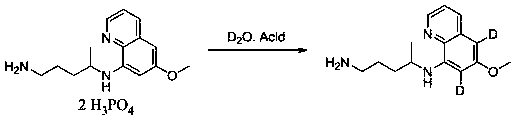Synthesis method of di-deuterated primaquine
A technology of primaquine and a synthetic method, which is applied in the field of deuterated drug production, can solve the problems of cumbersome operation, long route, high cost, etc., and achieve the effect of easy-to-obtain raw materials, high yield, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] To the three-necked flask, add primaquine phosphate (200 mg), heavy water (2 mL), add concentrated sulfuric acid (0.1 mL), heat to 95 °C, react for 8 h, pour into saturated sodium carbonate solution (20 mL) after the reaction is complete , extracted with ethyl acetate (20 mL x 2), dried over anhydrous sodium sulfate, and concentrated to obtain dideuterated primaquine (109 mg, 95% yield). 1 H NMR (300MHz, DMSO-d 6 ):8.21(dd, J = 4.5Hz, 1.2Hz, 1H), 8.01 (dd, J = 8.4Hz, 1.2Hz, 1H), 7.31 (dd, J =8.4Hz, 4.5Hz, 1H), 3.44(s, 3H), 3.19(m, 1H), 2.70(m, 2H), 1.40(m, 4H), 0.91(d, J = 6.0 Hz, 3H); 13 C NMR (75MHz, DMSO-d 6 ): 159.76, 144.28, 138.92, 138.07, 131.04, 124.54, 121.33, 55.60, 48.65, 39.33, 31.59, 23.26, 18.20.
Embodiment 2
[0019] To the three-necked flask, add primaquine phosphate (200 mg), heavy water (2 mL), add concentrated sulfuric acid (0.1 mL), heat to 55 °C, and react for 24 h. After the reaction is completed, pour it into saturated sodium carbonate solution (20 mL) , extracted with ethyl acetate (20 mL x 2), dried over anhydrous sodium sulfate, and concentrated to obtain dideuterated primaquine (103 mg, 90% yield).
Embodiment 3
[0021] To the three-necked flask, add primaquine phosphate (200 mg), heavy water (2 mL), add concentrated sulfuric acid (0.1 mL), heat to 110 °C, react for 4 h, pour into saturated sodium carbonate solution (20 mL) after the reaction is complete , extracted with ethyl acetate (20 mL x 2), dried over anhydrous sodium sulfate, and concentrated to obtain dideuterated primaquine (102 mg, 89% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com