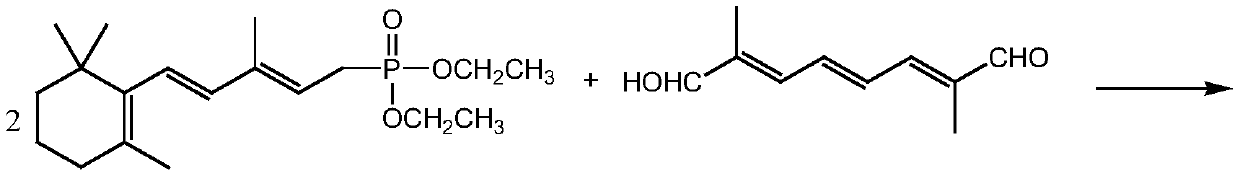Process method for synthesizing canthaxanthin
A process method, the technology of canthaxanthin, is applied in the production of bulk chemicals, organic chemistry, etc., and achieves the effects of low cost, easy industrial promotion, and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
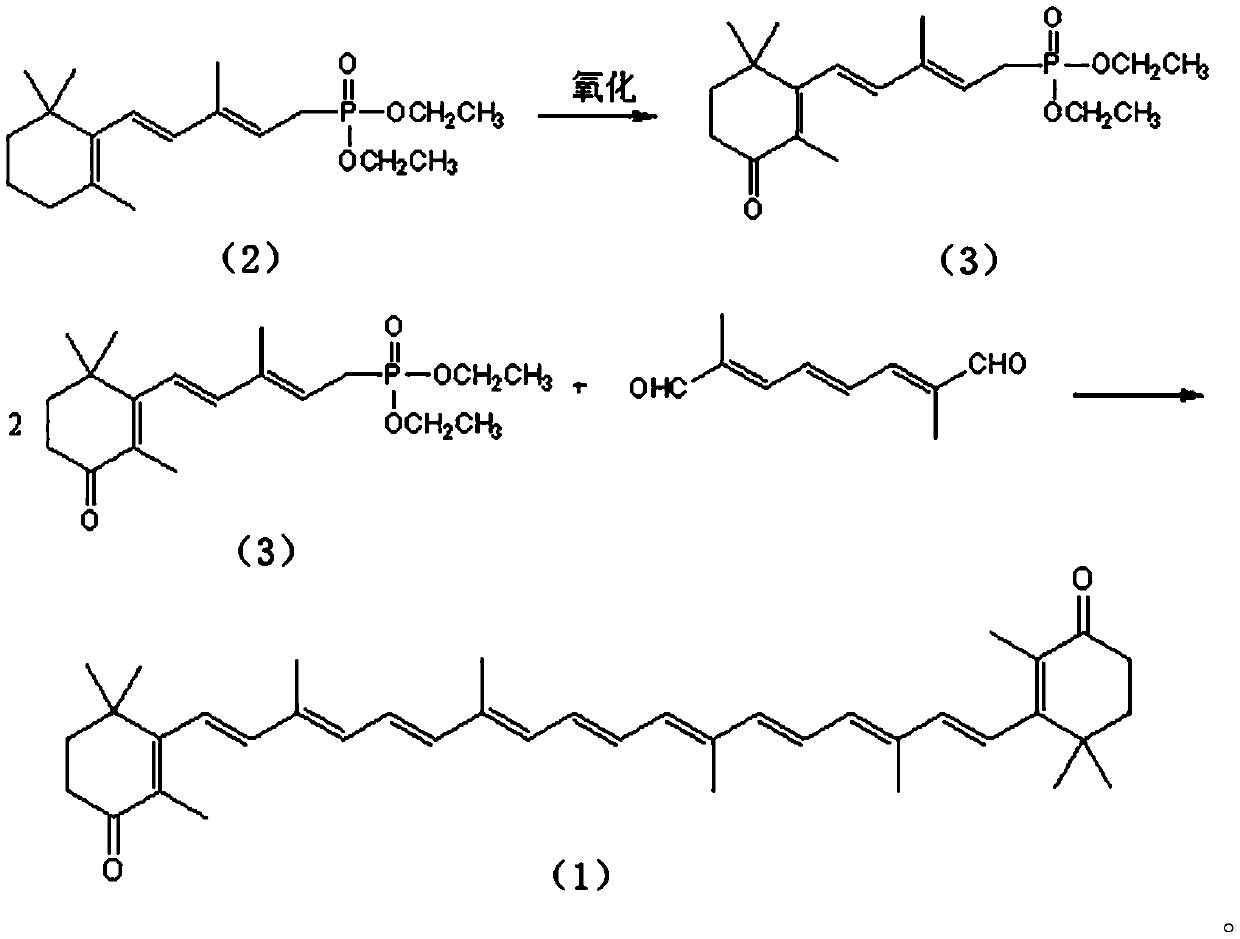
[0036] Compound (3) Preparation
[0037] Dissolve 34 grams (0.1 moles) of pentadecyl phosphate in 150 mL of toluene, maintain the reaction temperature at 10 ° C, then add commercially available Oxone (KHSO 5 Content is 47%) 32.4 grams (0.1 Mole), stirring reaction, gas phase follow-up reaction, when pentadecyl phosphate content is lower than 0.3%, add 100ml water to terminate reaction, divide and remove water phase, organic phase evaporates to dryness solvent and obtains 32.5 grams of product , the gas phase detection content was 97.6%, and the yield was 91.8%.
[0038] The product obtained in the present embodiment is carried out nuclear magnetic detection, and the results are as follows:
[0039] 1 H-NMR(δ,ppm)1.01(s,6H,-C(CH 3 ) 2 ),1.21(t,6H,-CH 3 ),1.49(t,2H,-CH 2 -),1.51(t,2H,-CH 2 -),1.71(s,3H,-CH 3 ), 1.83(d,3H,CH 3 C(3)),2.73(d,2H,-CH2-P),4.21(t,4H,OCH 2 ), 5.37-6.61 (m, 3H, -CH-); NMR results proved that the prepared product was compound (3).
Embodiment 2
[0041] Compound (3) preparation
[0042] Dissolve 34 grams (0.1 moles) of pentadecyl phosphate in 200 mL of dichloromethane, maintain the reaction temperature at 5°C, then add 8.15 grams (0.11 moles) of ketone peroxide, stir the reaction, and follow the reaction in the gas phase. When the phosphate ester content is lower than 0.3%, 250ml of water is added to terminate the reaction, the water phase is separated, the organic phase is evaporated to dryness to obtain 32.1 grams of product, the gas phase detection content is 96.5%, and the yield is 90.7%.
Embodiment 3
[0044] Compound (3) preparation
[0045] Dissolve 34 grams (0.1 moles) of pentadecyl phosphate in 200 mL of ethyl acetate, keep the reaction temperature at 15°C, then add 14.7 grams (0.13 moles) of 30% hydrogen peroxide, stir the reaction, follow the reaction in the gas phase, there is 25% The raw materials could not be reacted completely, 100ml of water was used to terminate the reaction, the water phase was separated, and the organic phase was evaporated to dryness. After the solvent was evaporated, 30 g of the product had a gas phase detection content of 98.5%, and a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com