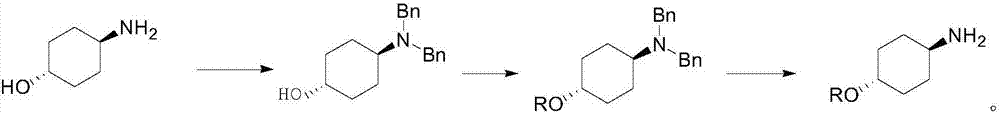
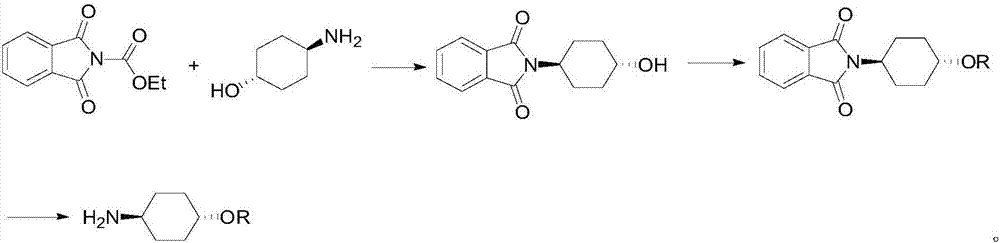
Preparation method for trans-4-alkoxycyclohexylamine
A technology of alkoxycyclohexylamine and hydroxycyclohexylamine, which is applied in the field of pharmaceutical intermediates, can solve the problems of high product loss, low product yield, and difficult operation, and achieve high total yield, environmental friendliness, and easy measurement produced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In this embodiment, trans-4-methoxycyclohexylamine is prepared by the following preparation method, which method includes the following steps:
[0060] (1) Preparation of the trans-4-hydroxycyclohexylamine of trityl protection:
[0061]
[0062] Trans-4-hydroxycyclohexylamine (115 g, 1 mol) was dissolved in dichloromethane (2 L), and triethylamine (121 g, 1.2 mol) was added with stirring, and the reaction system dropped to zero. Then trityl chloride (284g, 1.02mol) was added portion by portion, the reaction was slowly warmed up to room temperature and stirred for 5 hours, the reaction solution was washed with 10% citric acid, washed with saturated brine, dried over anhydrous sodium sulfate, and off-white Powder, crystallized with ethyl acetate petroleum ether to obtain 332g of pure product, yield 93%.
[0063] (2) Preparation of trans 4-methoxycyclohexylamine protected by trityl group:
[0064]
[0065] The compound obtained in step (1) (250 g, 0.7 mol) was diss...
Embodiment 2
[0071] In the present embodiment, trans-4-isopropoxycyclohexylamine is prepared by the following steps, the method comprising the following steps:
[0072] (1) Preparation of the trans-4-hydroxycyclohexylamine of trityl protection:
[0073]
[0074] Trans-4-hydroxycyclohexylamine (115 g, 1 mol) was dissolved in dichloromethane (2 L), and triethylamine (151.25 g, 1.5 mol) was added with stirring, and the reaction system dropped to zero. Then trityl chloride (278g, 1mol) was added portion by portion, the reaction was slowly warmed up to room temperature and stirred for 10 hours, the reaction solution was washed with 10% citric acid, washed with saturated brine, and dried over anhydrous sodium sulfate to obtain off-white powder , crystallized with ethyl acetate petroleum ether to obtain 327g of pure product, yield 91.6%.
[0075] (2) Preparation of trans 4-isopropoxycyclohexylamine protected by trityl group:
[0076]
[0077] The compound obtained in step (1) (250 g, 0.7 ...
Embodiment 3
[0083] In the present embodiment, trans-4-butoxycyclohexylamine is prepared through the following steps, the method comprising the following steps:
[0084] (1) Preparation of the trans-4-hydroxycyclohexylamine of trityl protection:
[0085]
[0086] Trans-4-hydroxycyclohexylamine (115 g, 1 mol) was dissolved in dichloromethane (2 L), and triethylamine (101 g, 1 mol) was added with stirring, and the reaction system dropped to zero. Then trityl bromide (505g, 1.5mol) was added portion by portion, the reaction was slowly warmed up to room temperature and stirred for 1 hour, the reaction solution was washed with 10% citric acid, washed with saturated brine, dried over anhydrous sodium sulfate, and off-white Powder, crystallized with ethyl acetate petroleum ether to obtain 306g of pure product, yield 85.7%.
[0087] (2) Preparation of the trans-4-butoxycyclohexylamine of trityl protection:
[0088]
[0089] The compound obtained in step (1) (250 g, 0.7 mol) was dissolved i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com