Novel method for preparing benzo 1,3-oxathiane-4-ketone
A technology of oxathione and a new method, which is applied in the field of preparation of benzo1,3-oxathione-4-one, can solve the problem that the yield is only 36%, the efficiency is not high, and it is irritating Problems such as smell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
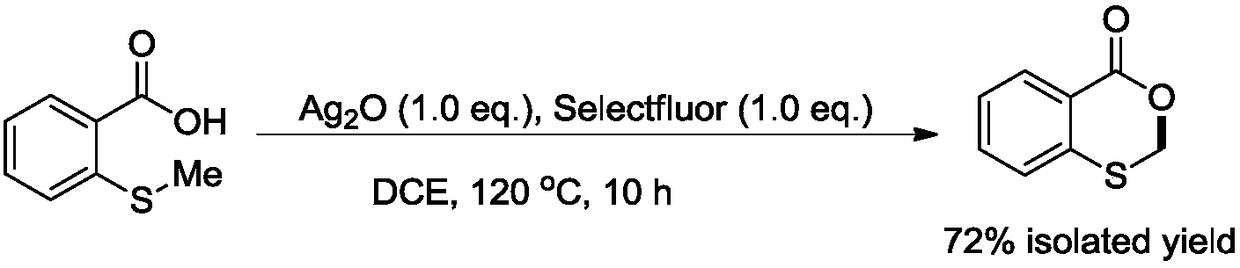
[0013] Add 2-carboxyphenyl methyl sulfide (1mmol, 0.17g), silver oxide (1mmol, 0.23g), 1-chloromethyl-4-fluoro-1,4-diazepine in sequence to a 100mL pressure tube Bicyclo[2.2.2]octane bis(tetrafluoroborate) salt (1mmol, 0.35g) and 1,2-dichloroethane (10mL) were reacted at 120°C with vigorous stirring for 10 hours. After the reaction, it was cooled to room temperature, and the reaction liquid was concentrated and separated by column chromatography in sequence to obtain benzo1,3-oxathiolane-4-one (0.12 g, 72%).
[0014] The equations involved in the reaction are as follows:
[0015]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com