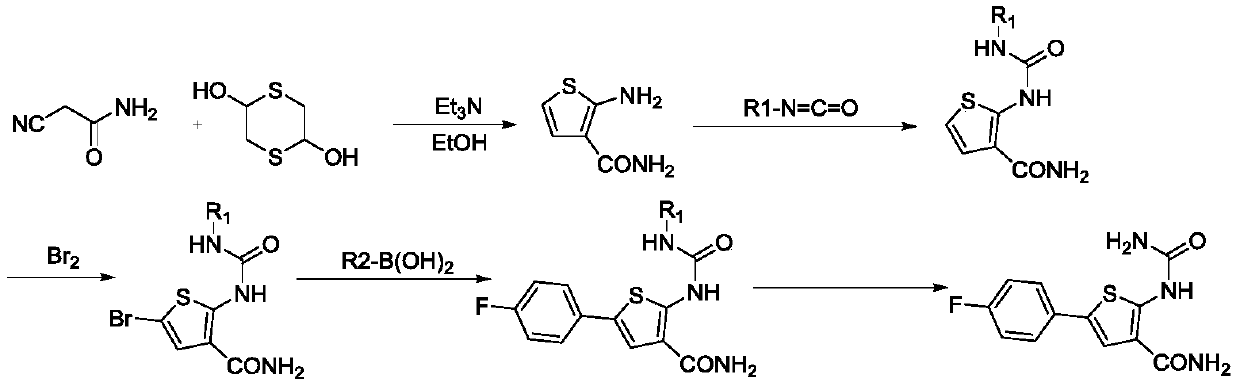
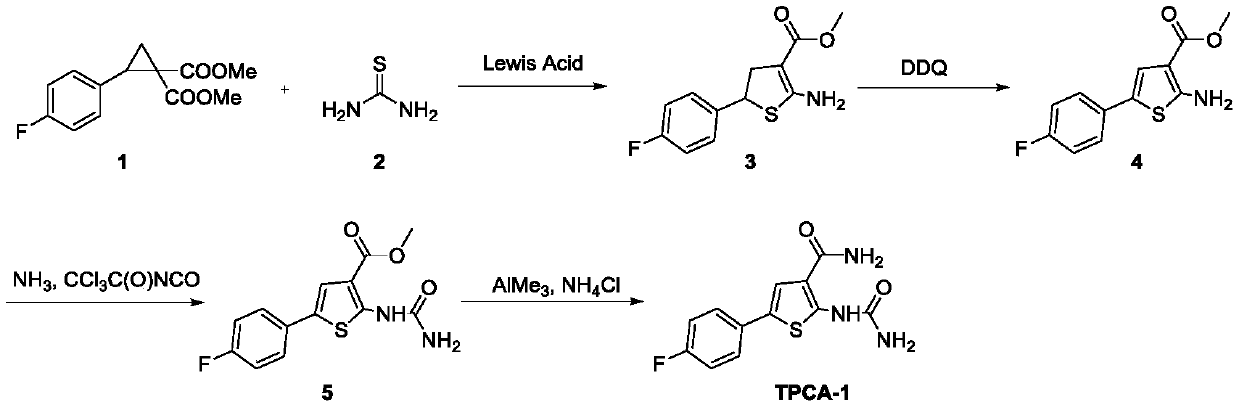
A kind of method of synthesizing thiophene inhibitor TPCA-1
A TPCA-1 and inhibitor technology, applied in the direction of organic chemistry, can solve the problems of cumbersome synthesis steps, harsh conditions, and inconvenient use, and achieve the effects of high yield, mild reaction conditions, and easy-to-obtain reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
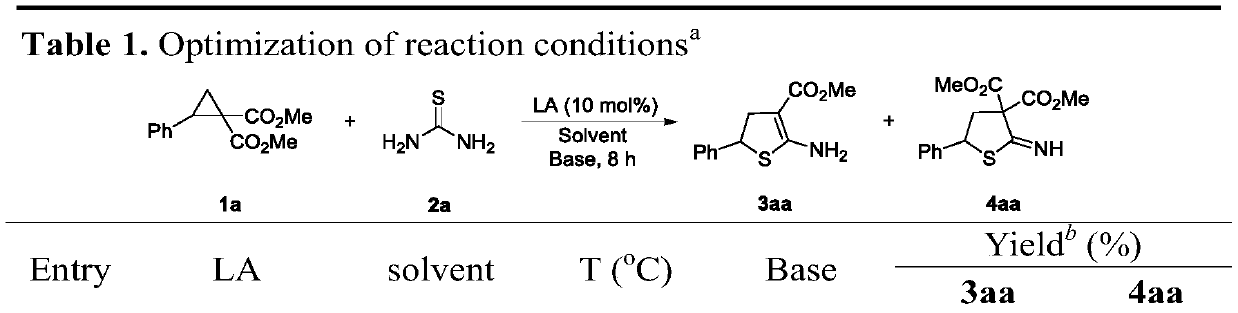
[0026] Optimization of ring expansion reaction conditions:
[0027]
[0028]
[0029] During the screening of reaction conditions, the effects of different catalysts, bases, solvents and temperatures on the reaction were investigated. Finalized Yb(OTf) 3 It is the best catalyst, dichloroethane is the best solvent, rubidium carbonate is the best base, and the reaction temperature is 90°C.
Embodiment 2
[0031] Step 1: Add 1(50.4mg, 0.2mmol), 2(30.4mg, 0.4mmol), Rb in turn to the reaction tube 2 CO 3 (9.2mg, 0.04mmol), Yb(OTf) 3 (25mg, 0.04mmol), and dichloroethane 3mL. The mixture was warmed to 90°C and stirred for 8 hours. Monitored by TLC. The reaction mixture was concentrated in vacuo, followed by column chromatography to obtain product 3 as a white solid, yield 72%, 36.4 mg.
[0032] 1 H NMR (400MHz, CDCl 3 ):δ7.41-7.34(m,2H),7.04-6.95(m,2H),6.12(br,2H),4.81(t,J=8.0,1H),3.69(s,3H),3.39(dd ,J=14.0,8.4Hz,1H),3.10(dd,J=14.4,7.2Hz,1H);
[0033] 13 C NMR (150MHz, CDCl 3 ): δ166.7, 163.2, 162.3, 161.5, 137.6 (d, J C-F =3.0Hz), 128.9(d, J C-F =9.0Hz), 115.7(d, J C-F =21.0Hz), 90.6, 50.7, 41.8.
[0034] HRMS: exact mass calcd for C12H12FNO2S(M+H)+: requires m / z 254.0646, found m / z 264.0645.
[0035] Step 2: Add 3 (50.6 mg, 0.2 mmol) and 2 mL of dichloromethane into the reaction tube. The mixture was moved to -20°C and stirred, and DDQ (34 mg, 0.15 mol) was weighed ...
Embodiment 3
[0047] Step 1: Add 1 (50.4 mg, 0.2 mmol), 2 (30.4 mg, 0.4 mmol), Na 2 CO 3 (4.2mg, 0.04mmol), Yb(OTf) 3 (25mg, 0.04mmol), and dichloroethane 3mL. The mixture was warmed to 90°C and stirred for 8 hours. Monitored by TLC. The reaction mixture was concentrated in vacuo, followed by column chromatography to obtain product 3 as a white solid, yield 61%, 30.8 mg.
[0048] 1 H NMR (400MHz, CDCl 3 ):δ7.41-7.34(m,2H),7.04-6.95(m,2H),6.12(br,2H),4.81(t,J=8.0,1H),3.69(s,3H),3.39(dd ,J=14.0,8.4Hz,1H),3.10(dd,J=14.4,7.2Hz,1H);
[0049] 13 C NMR (150MHz, CDCl 3 ): δ166.7, 163.2, 162.3, 161.5, 137.6 (d, J C-F =3.0Hz), 128.9(d, J C-F =9.0Hz), 115.7(d, J C-F =21.0Hz), 90.6, 50.7, 41.8.
[0050] HRMS: exact mass calcd for C12H12FNO2S(M+H)+: requires m / z 254.0646, found m / z 264.0645.
[0051] Step 2: Add 3 (50.6 mg, 0.2 mmol) and 2 mL of dichloromethane into the reaction tube. The mixture was moved to -20°C and stirred, and DDQ (34 mg, 0.15 mol) was weighed and slowly added to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com