Chiral 2-imidazoline aniline compound as well as preparation method and application thereof
A technology of imidazoline aniline and compound is applied in the field of chiral catalysts and achieves the effects of mild conditions, simple synthesis method and simple method
- Summary
- Abstract
- Description
- Claims
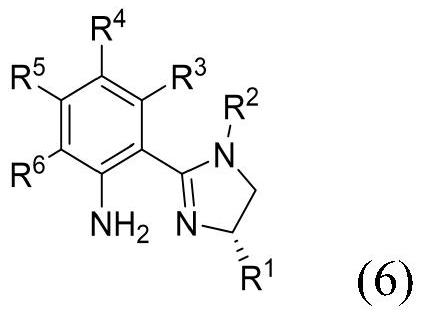
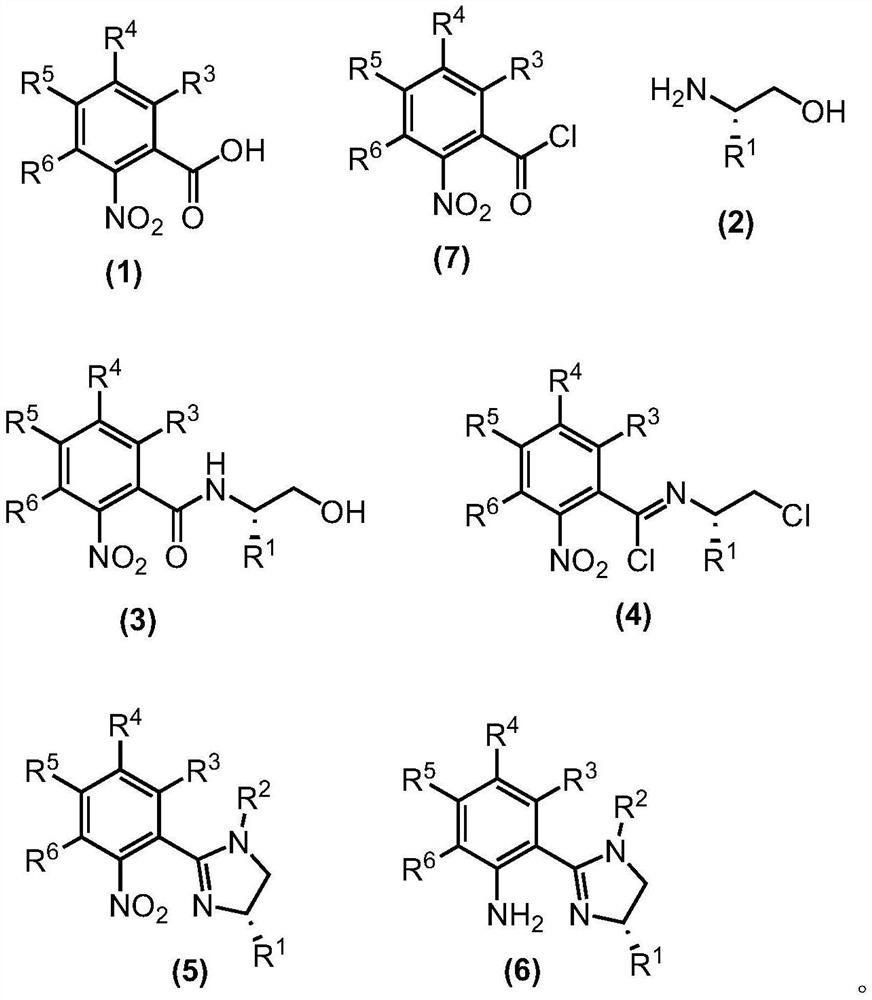
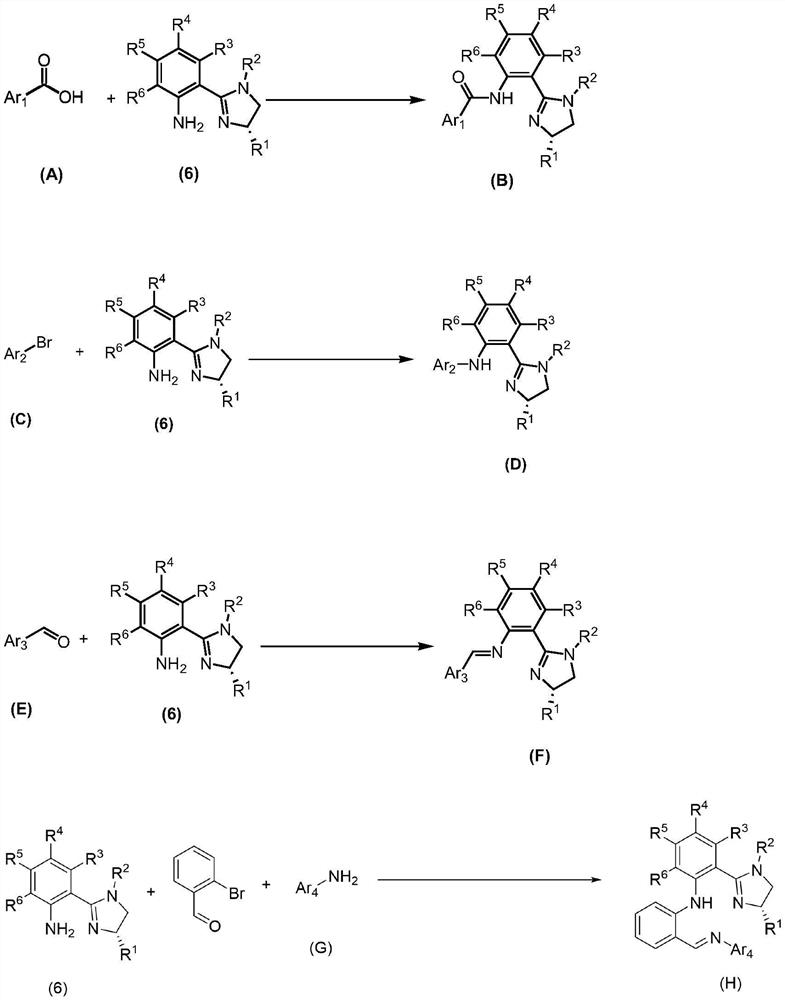
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Add 16.7g (100mmol) of o-nitrobenzoic acid, 100mL of dichloromethane and 2 drops of N,N-dimethylformamide in a 250mL round bottom flask, slowly add oxalyl chloride (150mmol) dropwise at room temperature, After reacting for 5-8 hours, dichloromethane and excess oxalyl chloride were removed under reduced pressure to obtain o-nitrobenzoyl chloride without purification; the preparation reaction equation is as follows:
[0056]
[0057] (2) Add 0.5151g (5mmol) S-valinol, 20mL dichloromethane and 1.4mL (10mmol) triethylamine into a 100mL round bottom flask, stir well, add 0.6mL (5mmol) o-nitrate under ice bath Base benzoyl chloride, after the addition, was warmed up to room temperature and stirred for 8 hours; the reaction solution was washed with water and saturated brine successively, separated after washing, and the organic phase was dried with anhydrous sodium sulfate, and concentrated to obtain N-[(1S)-2 -Methyl-1-hydroxymethylpropyl]-2-nitrobenzamide (3b), withou...
Embodiment 2
[0064] (1) With step (1) in embodiment 1.
[0065] (2) Same as step (2) in Example 1, wherein the difference is that 0.5151g S-valinol is changed to 0.5948g (5mmol) S-isoleucinol to obtain N-[(1S)-1-isoleucinol Butyl-2-hydroxyethyl]-2-nitrobenzamide crude product (3c), without purification; white solid; the preparation reaction equation is as follows:
[0066]
[0067] (3) With step (3) in embodiment 1, wherein difference is that the crude product of N-[(1S)-2-methyl-1-hydroxymethyl propyl]-2-nitrobenzamide is changed into N -[(1S)-1-Isobutyl-2-hydroxyethyl]-2-nitrobenzamide crude product to give 2-[(4S)-4-isobutyl-N-phenyl-2-imidazole Linyl] nitrobenzene crude product (5c), without purification; the preparation reaction equation is as follows:
[0068]
[0069] (5) Same as step (4) in Example 1, wherein the difference is that the crude product of [(4S)-4-isopropyl-N-phenyl-2-imidazolinyl]nitrobenzene is changed to [(4S) -4-isobutyl-N-phenyl-2-imidazolinyl]nitrobenzen...
Embodiment 3
[0072] (1) With step (1) in embodiment 1.
[0073] (2) With step (2) in embodiment 1, wherein different is that 0.5151g S-valinol is changed into 0.5948g (5mmol) S-secondary leucinol, obtains N-[(1S)-1-secondary Butyl-2-hydroxyethyl]-2-nitrobenzamide crude product (3d), without purification; white solid; the preparation reaction equation is shown in the following formula:
[0074]
[0075] (3) With step (3) in embodiment 1, wherein difference is that the crude product of N-[(1S)-2-methyl-1-hydroxymethyl propyl]-2-nitrobenzamide is changed into N -[(1S)-1-sec-butyl-2-hydroxyethyl]-2-nitrobenzamide crude to give 2-[(4S)-4-sec-butyl-N-phenyl-2-imidazole Linyl] nitrobenzene crude product (5d), without purification; the preparation reaction equation is shown in the following formula:
[0076]
[0077] (4) With step (4) in embodiment 1, wherein difference is that [(4S)-4-isopropyl-N-phenyl-2-imidazolinyl] nitrobenzene crude product is changed into [(4S) -4-sec-butyl-N-pheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com