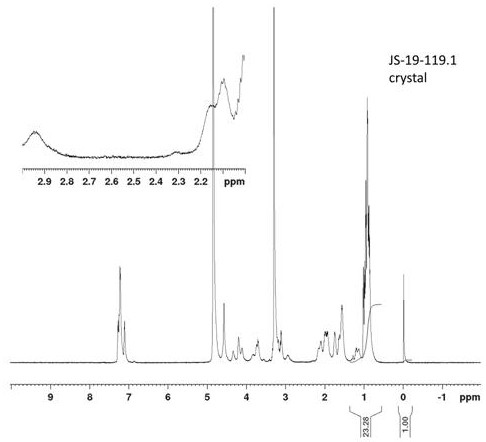
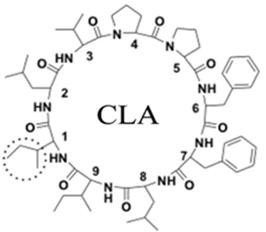
A method for preparing high-purity cyclic peptide monomers by generating cyclic peptide crystals
A high-purity, cyclic peptide technology, applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of high operating costs, production costs, and high equipment requirements, and achieve low cost, simple preparation process, and production conditions. easily controlled effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for preparing high-purity cyclic peptide monomers by generating cyclic peptide crystals comprises the following steps.
[0034] (1) Dissolve the crude cyclic peptide solid powder in pure methanol according to the mass volume ratio of the crude cyclic peptide solid powder to pure methanol: 1g:16ml, centrifuge at 4500 r / min for 7 min, and collect the supernatant liquid.
[0035] (2) Rotate the supernatant to dryness at a temperature of 50° C. to obtain a preliminarily purified solid product.
[0036] (3) Add pure methanol dropwise to the obtained preliminary purified solid product according to the ratio of the mass volume ratio of the preliminary purified solid product to the final added pure methanol: 1g:4.2ml, and add methanol at a water bath temperature of 50°C dissolved until a saturated solution was obtained.
[0037] (4) Keep the temperature of the 50°C water bath in step (3) constant, and add distilled water dropwise to the saturated solution obtained in...
Embodiment 2
[0040] A method for preparing high-purity cyclic peptide monomers by generating cyclic peptide crystals comprises the following steps.
[0041] (1) Dissolve the crude cyclic peptide solid powder in pure methanol according to the mass volume ratio of the crude cyclic peptide solid powder to pure methanol: 1g:12.5ml, centrifuge at 3000 r / min for 10min, and collect the supernatant liquid.
[0042] (2) The supernatant was rotatably concentrated to dryness at a temperature of 40° C. to obtain a preliminarily purified solid product.
[0043] (3) Add pure methanol dropwise to the obtained preliminary purified solid product according to the ratio of the mass volume ratio of the preliminary purified solid product to the final added pure methanol: 1g:3.5ml, and add methanol at a water bath temperature of 60°C dissolved until a saturated solution was obtained.
[0044] (4) Keep the temperature of the water bath in step (3) constant, add distilled water drop by drop to the saturated sol...
Embodiment 3
[0047] A method for preparing high-purity cyclic peptide monomers by generating cyclic peptide crystals comprises the following steps.
[0048] (1) Dissolve the crude cyclic peptide solid powder in pure methanol according to the mass volume ratio of the crude cyclic peptide solid powder to pure methanol: 1g:20ml, centrifuge at 4000 r / min for 15min, and collect the supernatant .
[0049] (2) The supernatant was rotatably concentrated to dryness at a temperature of 45° C. to obtain a preliminarily purified solid product.
[0050] (3) Add pure methanol dropwise to the obtained preliminary purified solid product according to the ratio of the mass volume ratio of the preliminary purified solid product to the final added pure methanol: 1g:5ml, and add methanol while adding methanol at a water bath temperature of 45°C. Dissolve until a saturated solution is obtained.
[0051] (4) Keep the temperature of the water bath in step (3) constant, add distilled water drop by drop to the sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com