Electroplating pearl nickel additive, electroplating pearl nickel solution and electroplating method thereof
A technology of nickel plating solution and pearl nickel, which is applied in the field of pearl nickel, can solve the problems of low production efficiency and high cost, and achieve the effects of low consumption, simple types and stable plating solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
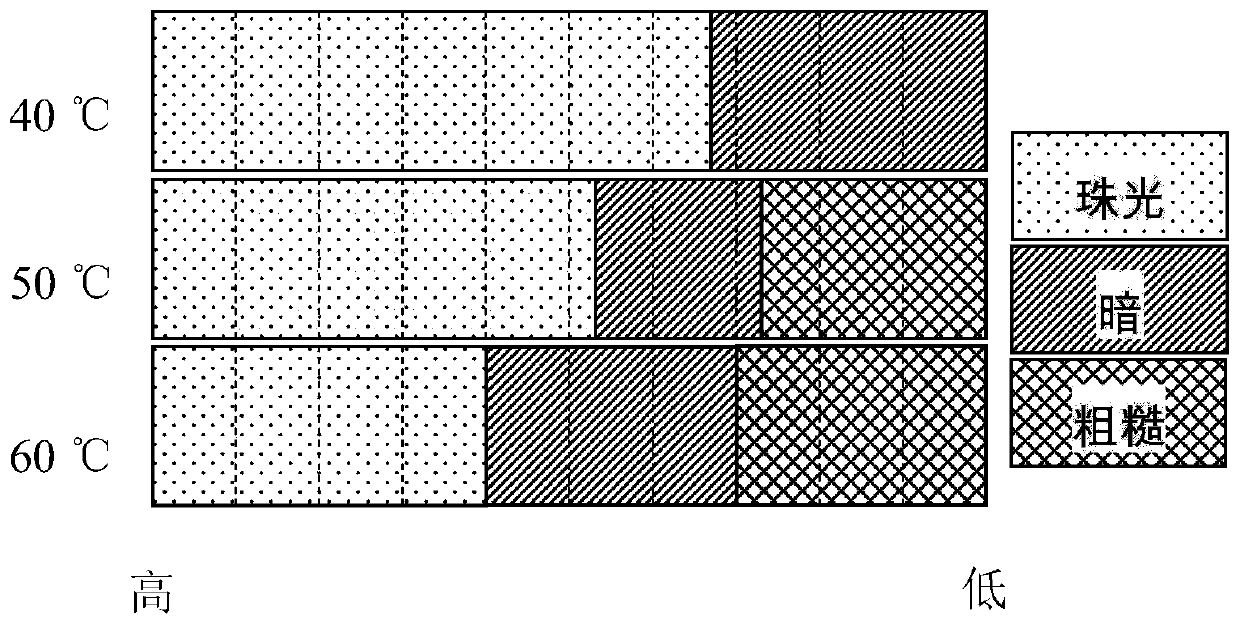
[0032] Add 6.0g / L sodium dicyanamide (Na[DCA]) additive to the solution containing 170g / LNiSO 4 ·6H 2 O, 25g / LH 3 BO 3 in the aqueous solution. The effect of current density and temperature on pearl nickel coating was investigated by Hall cell experiment. According to the formula j=i(5.1-5.42lgl), the current density from the proximal end to the distal end is changed from 0.3A / dm 2 Rise to 15.3A / dm 2 , at different temperatures, the coating condition of the Hall cell is as follows figure 1 shown.
[0033] from figure 1 It can be seen that at 40°C, when the current density is greater than 2A / dm 2 When the nickel coating has white pearlescent properties, when the current density is less than 2A / dm 2 , the color of the coating becomes darker, grayish black. As the temperature increases, the pearlescent region narrows and the film becomes rough in the low current density region.
Embodiment 2
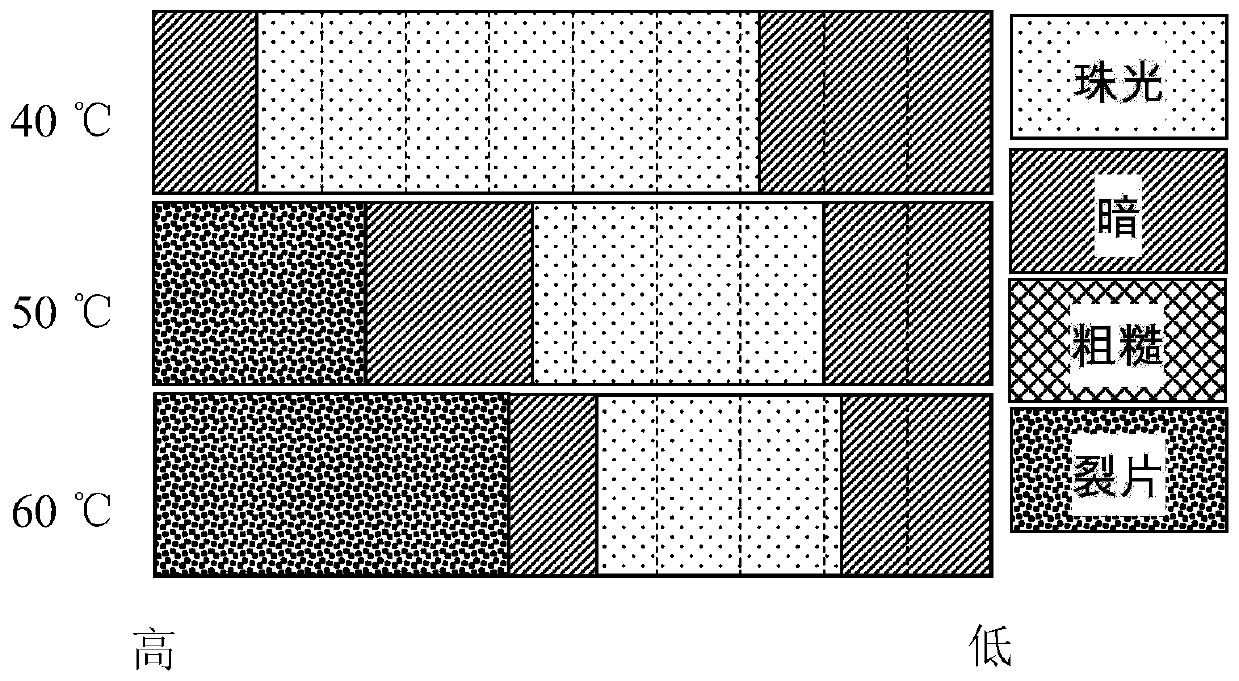
[0035] Add 1.0g / L sodium dicyanamide (Na[DCA]) additive to the solution containing 170g / LNiSO 4 ·6H 2 O, 25g / L H 3 BO 3 in the aqueous solution. The effect of current density on pearl nickel coating was investigated by Hall cell experiment. According to the formula j=i(5.1-5.42lgl), the current density from the proximal end to the distal end is changed from 0.3A / dm 2 Rise to 15.3A / dm 2 , at different temperatures, the coating condition of the Hall cell is as follows figure 2 shown. At 40°C, at a current density of 3-11A / dm 2 The coating in the interval presents white pearlescent. As the temperature rises, the pearlescent region narrows and moves to the low current density region. Cracks occur in the plating layer in the high current density area.
Embodiment 3
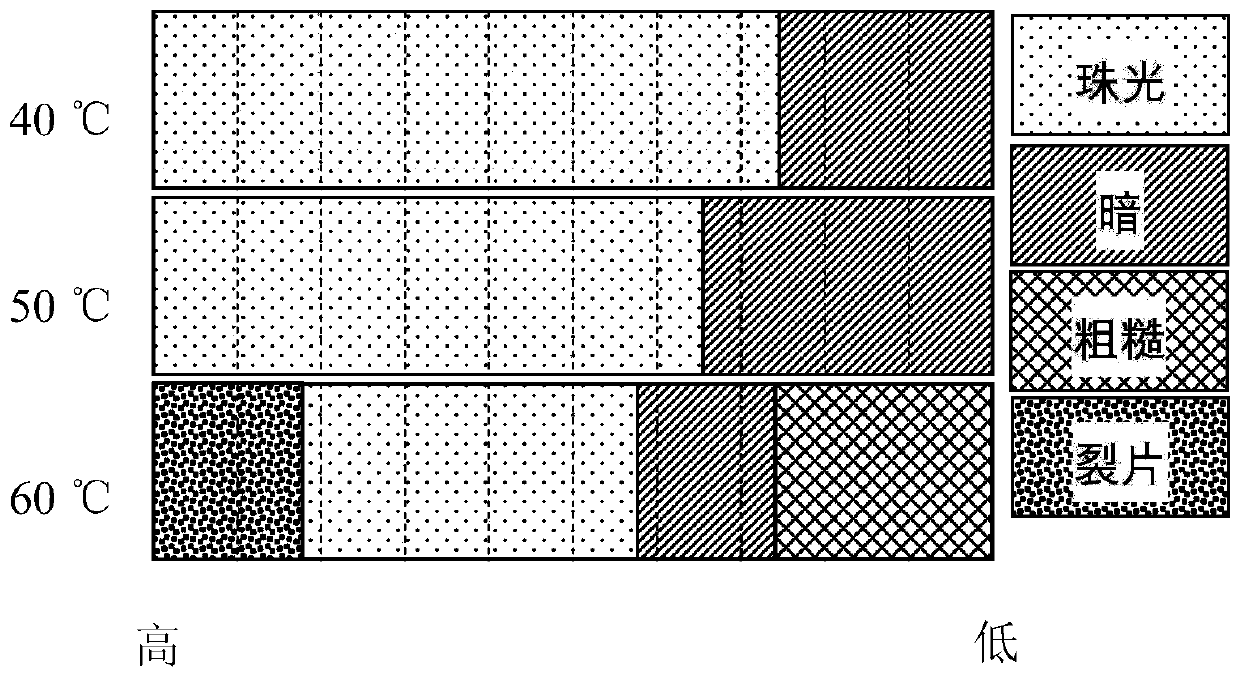
[0037] 4.0g / L sodium dicyanamide additive is added to containing 330g / LNiSO 4 ·6H 2 O, 37g / L H 3 BO 3 in the aqueous solution. The effect of current density on pearl nickel coating was investigated by Hall cell experiment. According to the formula j=i(5.1-5.42lgl), the current density from the proximal end to the distal end is changed from 0.3A / dm 2 Rise to 15.3A / dm 2 , at different temperatures, the coating condition of the Hall cell is as follows image 3 shown. Pearlescent nickel exhibits white pearlescence in the high current density area, and the coating color becomes darker in the low current density area, showing gray black. As the temperature of the plating solution increases, the pearlescent region becomes narrower. At 60°C, the current density is higher than 11A / dm 2 , the coating cracks.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com