Preparation method of fimasartan impurities
A Fimasartan and impurity technology, which is applied in the field of chemical substance preparation, can solve the problems of no preparation method and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]A preparation method of Fimasartan impurity E, comprising the steps of: adding Lawesson reagent to the FMST-1 toluene solution with a concentration of 0.17g / ml in a nitrogen atmosphere, heating up to 70°C, and stirring for 5h to obtain a reaction solution , vacuum-dry the reaction solution to obtain the crude product, then add ethyl acetate 5 times the weight of the crude product and water 5 times the weight of the crude product, heat up to dissolve, reflux, add activated carbon, reflux 1h, slowly cool to room temperature, stir 2h, filter, rinse the filter cake with ethyl acetate, adjust the temperature to 45°C, and blow dry for 6h to obtain impurity E, wherein the amount of Lawesson reagent is 0.6eq, the weight ratio of FMST-1 and activated carbon is 26.07:3, the impurity The yield of E is 63.8%;
[0044] The impurity E was detected by proton nuclear magnetic spectrum, and the detection result of proton nuclear magnetic spectrum was: 1H-NMR (400Mz, CDCl3) δ: 7.850~7.827...
Embodiment 2
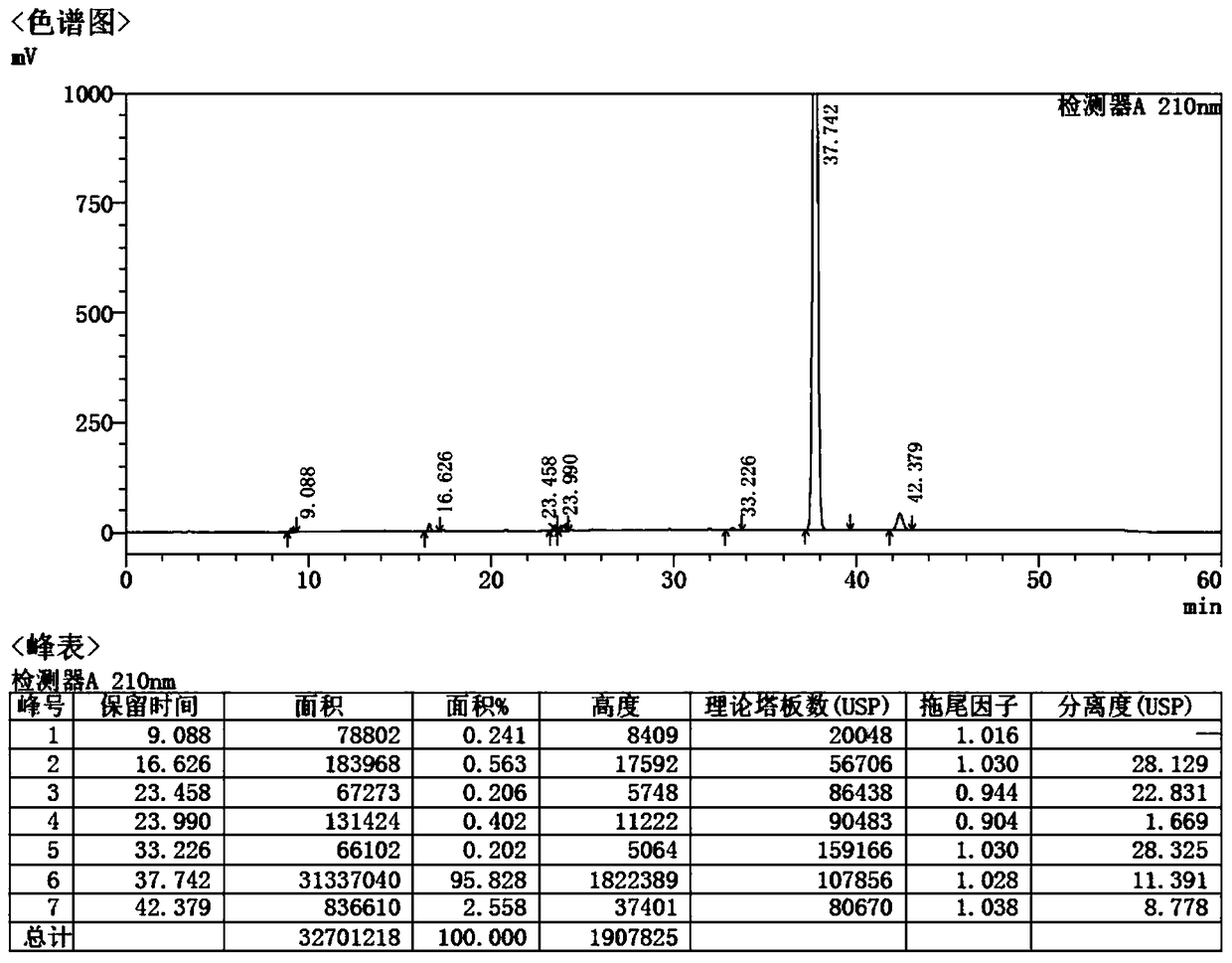
[0051] A preparation method of Fimasartan impurity K, comprising the steps of: adding Lawesson reagent to the FMST-1 toluene solution with a concentration of 0.17g / ml in a nitrogen atmosphere, heating up to 70°C, and stirring for 5h to obtain a reaction solution , the reaction solution was evaporated to dryness in vacuo to obtain the crude product, the crude product was subjected to column chromatography, the eluate was collected, the temperature was adjusted to 40°C, and the impurity K was obtained by vacuum drying, wherein the mobile phase of the column chromatography was a mixture of methanol and dichloromethane Solution, wherein, the volume ratio of methanol and dichloromethane is 1:15, the amount of Lawesson reagent is 1.2eq, the weight volume (g / L) ratio of FMST-1 and eluent is 25.88:1, the yield of impurity K 68.3%;
[0052] The impurity K is detected by proton nuclear magnetic spectrum, and the proton nuclear magnetic spectrum detection result is: 1H-NMR (400Mz, CDCl3)...
Embodiment 3
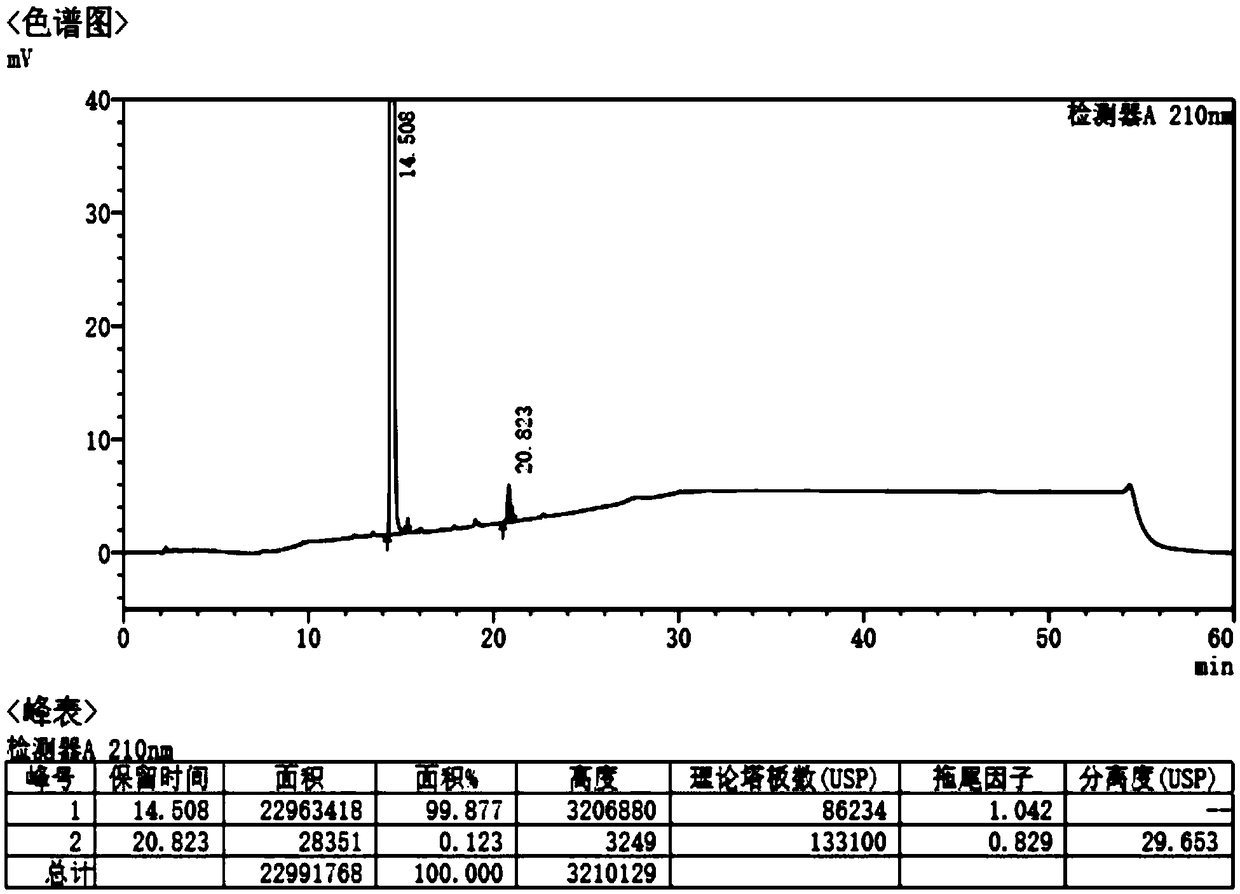
[0056] A preparation method of Fimasartan impurity E, comprising the steps of: adding Lawesson reagent to the FMST-1 acetonitrile solution with a concentration of 0.13 g / ml in a nitrogen atmosphere, heating up to 80° C., and stirring for 4 hours to obtain a reaction solution , the reaction solution was vacuum-dried to obtain the crude product, then adding ethyl acetate 5 times the weight of the crude product and water 5 times the weight of the crude product, heating up to dissolve, reflux, adding activated carbon, refluxing for 0.5h, slowly cooling to room temperature, Stir for 3h, filter, rinse the filter cake with ethyl acetate, adjust the temperature to 50°C, and blow dry for 8h to obtain impurity E, wherein the amount of Lawesson reagent is 0.3eq, and the weight ratio of FMST-1 to activated carbon is 26.07:3, The yield of impurity E was 60.5%, and the purity of impurity E by HPLC analysis was 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com