Application of ligustilide to preparation of drug for preventing and treating osteoporosis
A technology for osteoporosis and ligustilide, which is applied in the application field of ligustilide in the preparation of medicines for preventing and treating osteoporosis, and achieves the effect of improving bone formation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
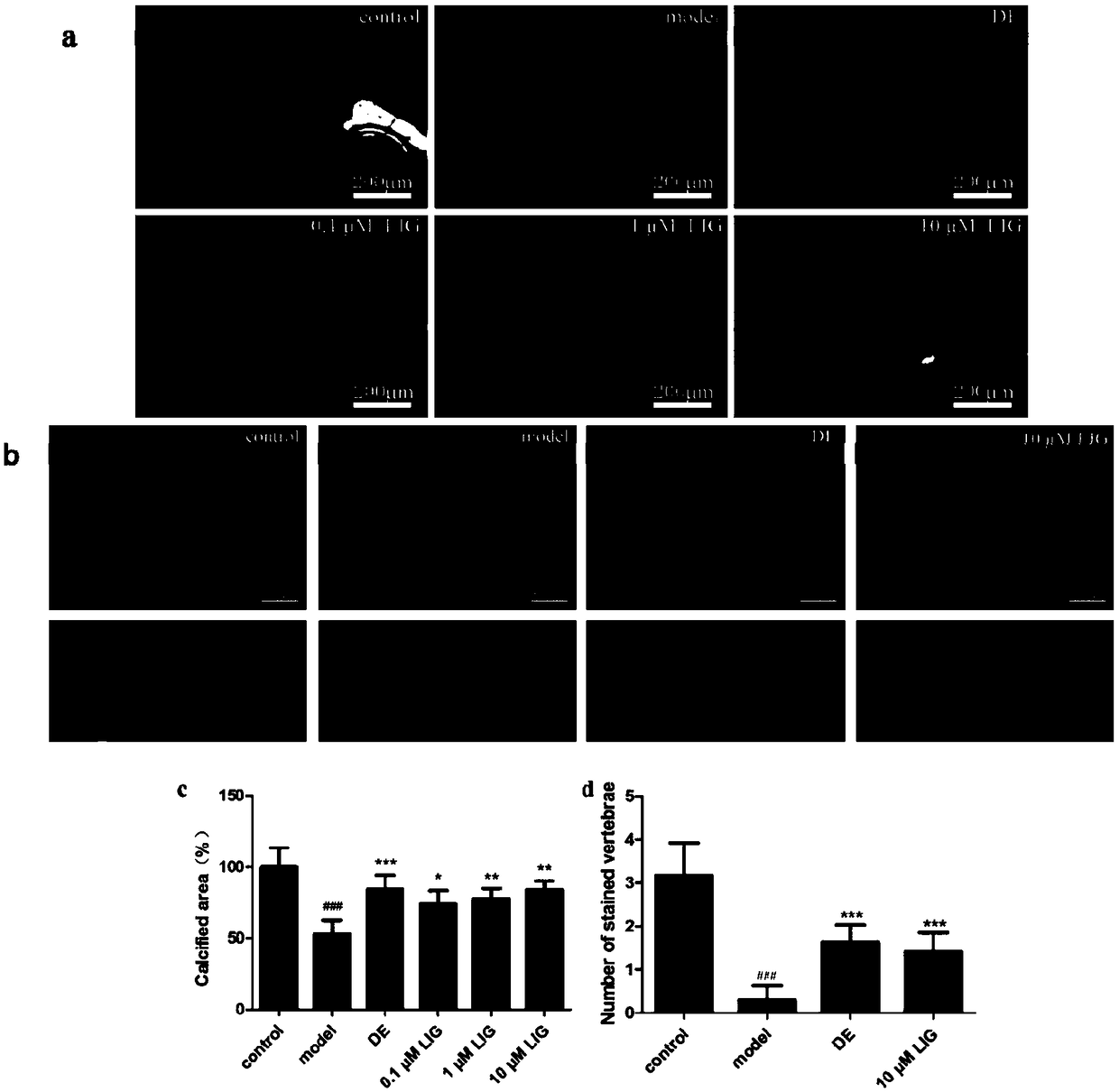
[0027] Effects of ligustilide on prednisolone-induced zebrafish osteoporosis
[0028] Experimental animals: zebrafish AB strain was purchased from Kaiji Biotechnology Co., Ltd.; circadian rhythm: day:night=14h:10h; temperature: (28.5±0.5)°C.
[0029] Reagents and drugs: prednisolone was purchased from Shanghai Yuanye Biotechnology Co., Ltd.; etidronate disodium was purchased from Shanghai Yuanye Biotechnology Co., Ltd.
[0030] Instrument: Leica M205FA stereo fluorescence microscope, etc.
[0031] Grouping and administration: divided into control group (0.2% DMSO), model group model (25 μM prednisolone), positive drug group DE (15 μg / ml etidronate disodium), ligustilide group LIG (0.1, 1 and 10 μM)
[0032] Experimental method: Beginning at 3 days post fertilization (dpf), zebrafish larvae were treated with 25 μM prednisolone for 6 consecutive days. Two days after modeling, etidronate disodium (DE; 15 μg / ml) was used as a positive drug, or ligustilide (0.1, 1 and 10 μM) was...
Embodiment 2
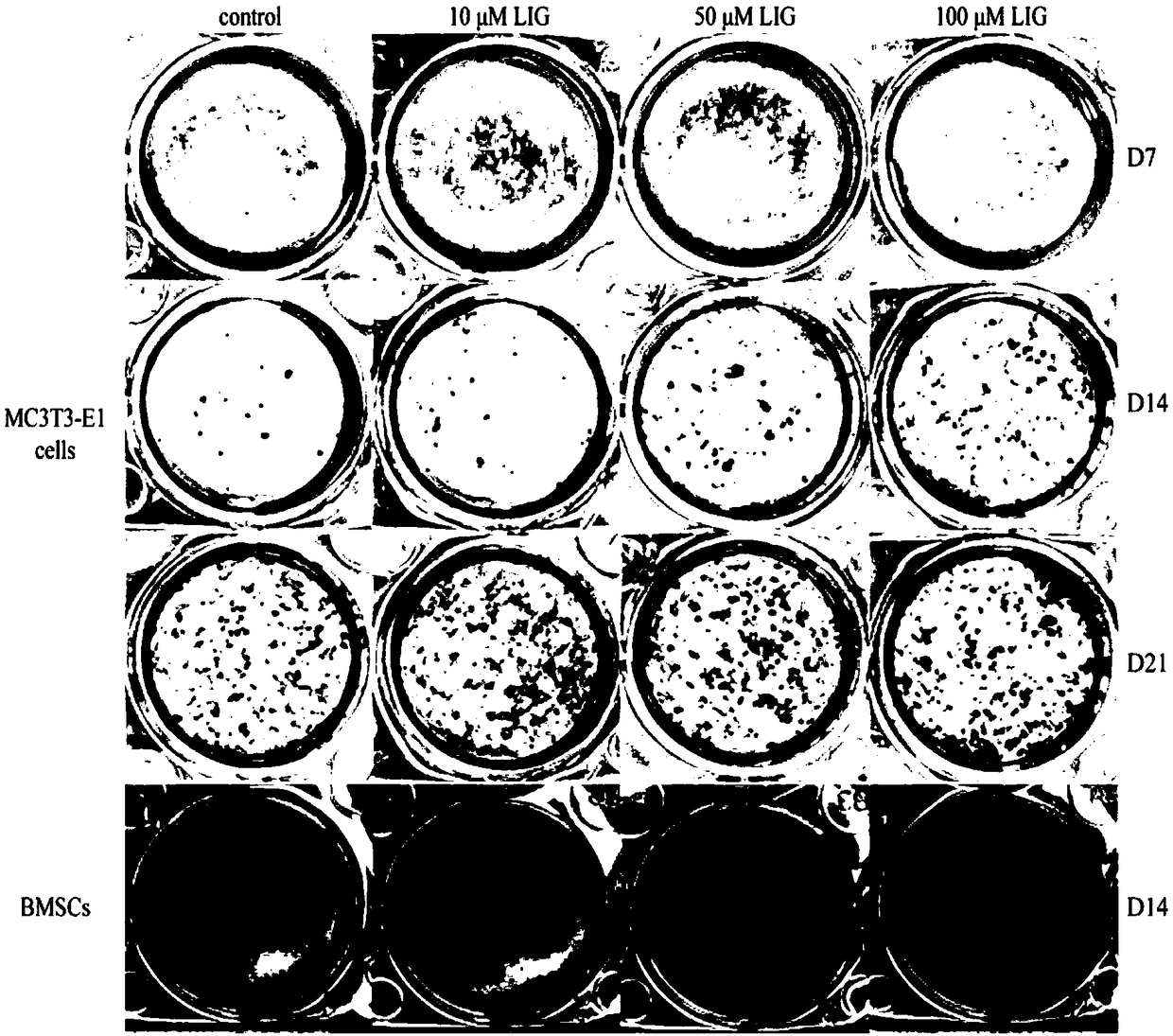
[0036] Effects of Ligustilide on Osteoblast Differentiation
[0037] Cells: The pre-osteoblast cell line MC3T3-E1 was purchased from the Cell Bank of the Shanghai Academy of Chinese Sciences, and bone marrow mesenchymal stem cells (BMSCs) derived from rat femurs were self-extracted.
[0038] Reagents and medicines: β-glycerophosphate was purchased from Sigma; dexamethasone was purchased from Sigma; ascorbic acid was purchased from Sigma.
[0039] Instrument: Leica stereo microscope, etc.
[0040] Grouping and administration: divided into blank group (0.1%DMSO), ligustilide group (10, 50 and 100 μM)
[0041] Experimental method: use α-MEM culture medium containing 10% heat-inactivated FBS, and add double antibodies to the culture medium at a dilution ratio of 1:100, at 37°C, 5% CO 2 , cultured in a saturated humidity incubator. Medium was changed every two days. When the cells grow to about 90%, trypsinize the cells into a centrifuge tube. Centrifuge at 1000 rpm for 3 min....
Embodiment 3
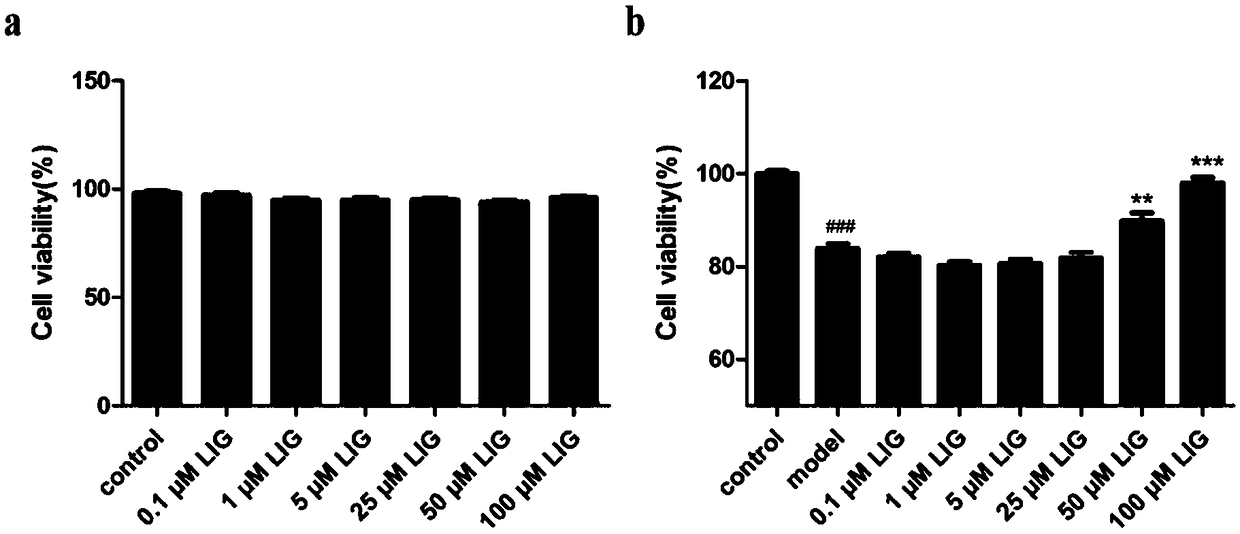
[0044] Effects of ligustilide on the survival rate of MC3T3-E1 cells under oxidative stress
[0045] Cells: Pre-osteogenic cell line MC3T3-E1 was purchased from the Cell Bank of Shanghai Academy of Chinese Sciences.
[0046] Instrument: Bio-Tek microplate reader.
[0047] Grouping and administration: divided into blank control group control (0.1% DMSO), model group model (0.4 mM H 2 o 2 ), ligustilide group LIG (1, 10, 50, 100 μM).
[0048] Experimental method: use α-MEM culture medium containing 10% heat-inactivated FBS, and add double antibodies to the culture medium at a dilution ratio of 1:100, at 37°C, 5% CO 2 , cultured in a saturated humidity incubator. Medium was changed every two days. When the cells grow to about 90%, trypsinize the cells into a centrifuge tube. Centrifuge at 1000 rpm for 3 min. After centrifugation, the cells were resuspended in α-MEM culture medium and counted, with 200 μl of culture medium per well, 1×10 4 Each well was seeded in a 96-well...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com