Na-K-Mg-Ca glucose injection and preparation method
A technology of glucose injection and glucose, applied in the field of sodium potassium magnesium calcium glucose injection and preparation, can solve the problems that patients are prone to cause acidosis, liver burden and the like, achieve the protection of liver and kidney function, correct acidosis, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
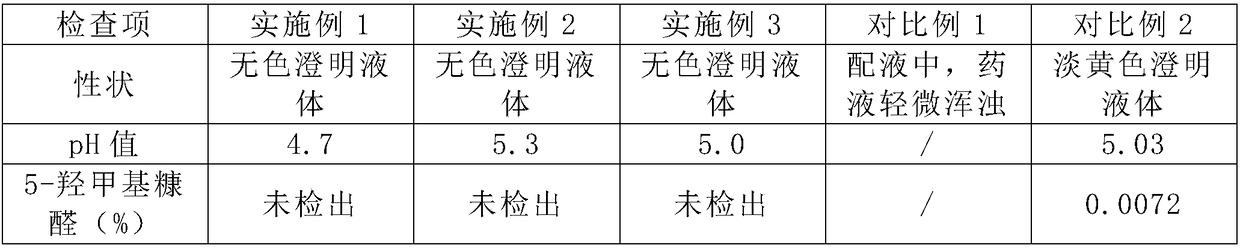
[0022] Embodiment 1: the preparation of sodium potassium magnesium calcium glucose injection comprises the following steps:
[0023] S1: Weigh 40% of the total amount of water for injection in the dilute preparation tank, cool down to 70°C-80°C, add 100g of glucose monohydrate, stir to dissolve, and transfer to a constant volume tank;
[0024] S2: Weigh 40% of the total amount of water for injection in the dilute tank, cool down to 50°C-60°C, add 0.876g of sodium chloride, 0.748g of potassium chloride, 0.304g of magnesium chloride hexahydrate, 1.12g of calcium gluconate, Potassium dihydrogen phosphate 1.36g, stir well;
[0025] S3: Add an appropriate amount of citric acid, stir to dissolve, then add 2.72 g of sodium acetate trihydrate, stir to dissolve, transfer to a constant volume tank, and lower the temperature of the constant volume tank to below 50°C;
[0026] S4: Add citric acid to adjust the pH of the solution to 4.7, and add water for injection to 1000 mL;
[0027] S...
Embodiment 2
[0031] Embodiment 2: the preparation of sodium potassium magnesium calcium glucose injection comprises the following steps:
[0032] S1: Weigh 40% of the total amount of water for injection in the dilute preparation tank, cool down to 70°C-80°C, add 100g of glucose monohydrate, stir to dissolve, and transfer to a constant volume tank;
[0033] S2: Weigh 40% of the total amount of water for injection in the dilute tank, cool down to 50°C-60°C, add 0.876g of sodium chloride, 0.748g of potassium chloride, 0.304g of magnesium chloride hexahydrate, 1.12g of calcium gluconate, Potassium dihydrogen phosphate 1.36g, stir well;
[0034] S3: Add an appropriate amount of citric acid, stir to dissolve, then add 2.72 g of sodium acetate trihydrate, stir to dissolve, transfer to a constant volume tank, and lower the temperature of the constant volume tank to below 50°C;
[0035] S4: Add citric acid, adjust the pH value of the solution to 5.3, and add water for injection to 1000 mL;
[003...
Embodiment 3
[0040] Embodiment 3: the preparation of sodium potassium magnesium calcium glucose injection comprises the following steps:
[0041] S1: Weigh 40% of the total amount of water for injection in the dilute preparation tank, cool down to 70°C-80°C, add 100g of glucose monohydrate, stir to dissolve, and transfer to a constant volume tank;
[0042] S2: Weigh 40% of the total amount of water for injection in the dilute tank, cool down to 50°C-60°C, add 0.876g of sodium chloride, 0.748g of potassium chloride, 0.304g of magnesium chloride hexahydrate, 1.12g of calcium gluconate, Potassium dihydrogen phosphate 1.36g, stir well;
[0043] S3: Add an appropriate amount of citric acid, stir to dissolve, then add 2.72 g of sodium acetate trihydrate, stir to dissolve, transfer to a constant volume tank, and lower the temperature of the constant volume tank to below 50°C;
[0044] S4: Add citric acid, adjust the pH value of the solution to 5.0, and replenish water for injection to 1000 mL; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com