L-phenylalanine modified maslinic acids as well as synthesis methods and application thereof
A synthesis method and technology of phenylalanine, applied in the field of maslinic acid derivatives, can solve the problems of undiscovered maslinic acid benzyl ester, etc., and achieve good proliferation inhibitory activity and good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
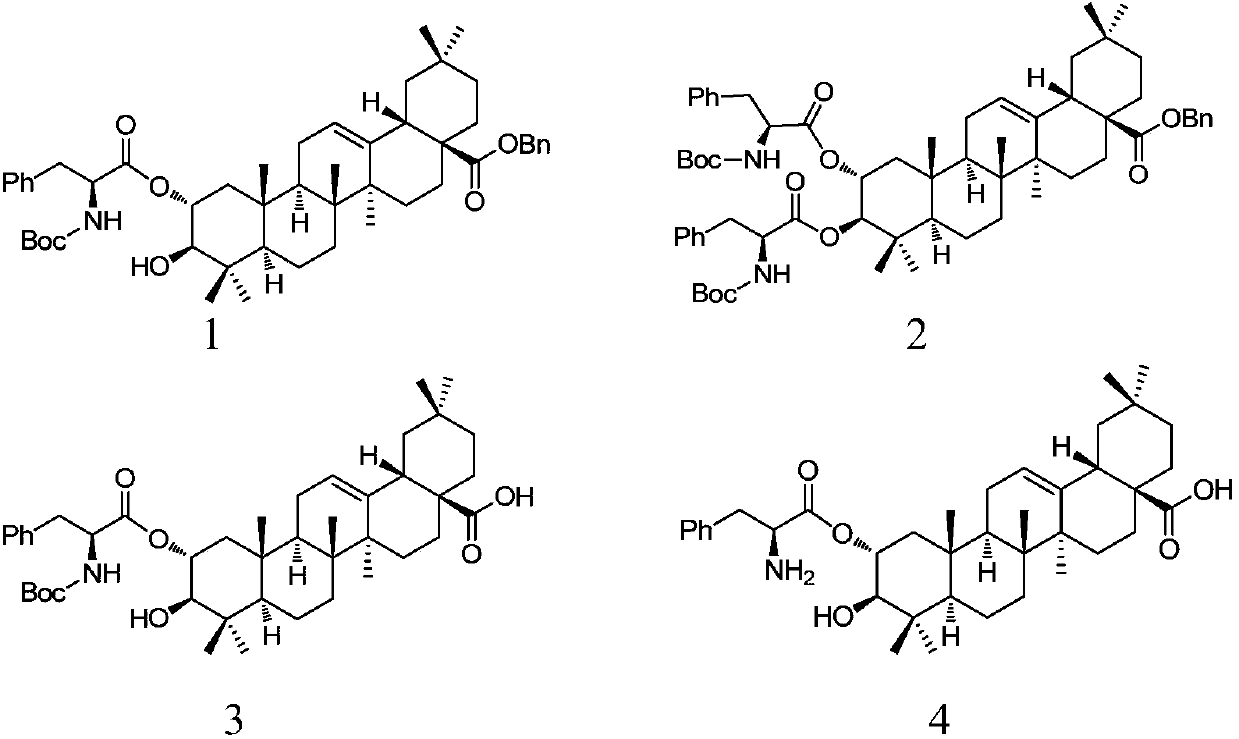
[0035] Embodiment 1: the synthesis of compound 1 and 2
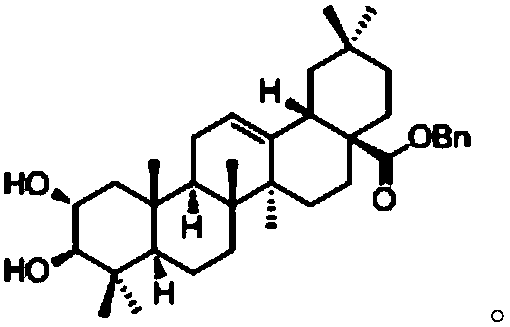
[0036] Get benzyl maslinic acid (3.00g, 5.33mmol) and dissolve in CH 2 Cl 2 (50mL), sequentially added Boc-L-phenylalanine (3.50g, 13.33mmol), DCC (3.00g, 13.33mmol) and DMAP (0.16g, 1.33mmol), stirred at room temperature for 12h, the reaction solution was filtered and washed with CH 2 Cl 2 After washing, the filtrate was evaporated under reduced pressure to obtain a residue, and the residue was separated by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =20:1 to 8:1), the combined fractions were detected by thin layer chromatography to obtain compound 1 (1.20g, 27.8%, white solid) and compound 2 (2.90g, 51.5%, white solid), respectively.
[0037] Compound 1
[0038]
[0039] Yield: 1.20g, 27.8%, white solid; R f =0.412 (Petroleum ether: EtOAc=5:1).m.p.96-98°C. 1 H NMR (400MHz, CDCl 3)6 (ppm): 0.58, 0.84, 0.90, 0.93, 0.96, 1.03, 1.11 (7s, each 3H, 7×CH 3 ), 1.41(s, 9H, 3×CH 3 Boc ), 0.71-2.05(m, 20H), 2.91...
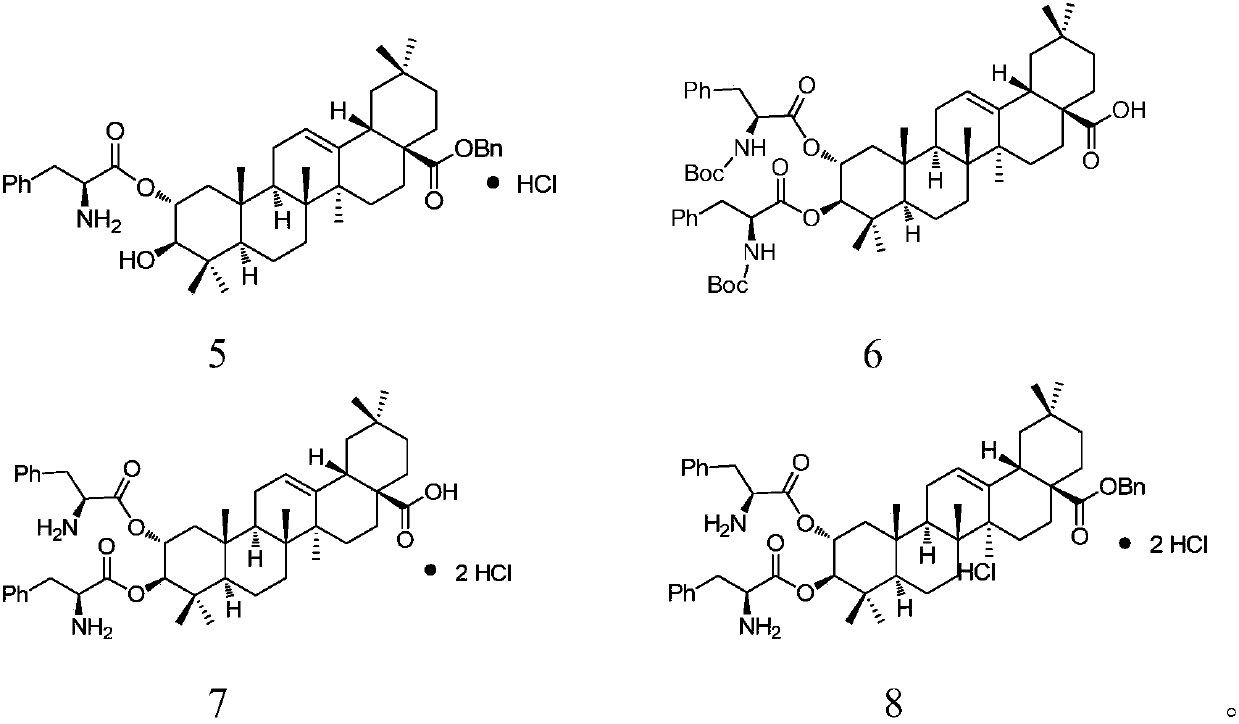
Embodiment 2
[0042] Embodiment 2: the synthesis of compound 3
[0043]
[0044] 1 (0.50g, 0.62mmol) was dissolved in methanol (30mL), ammonium formate (0.39g, 6.20mmol) and 10% Pd / C (0.05g) were added successively, and reacted at 55°C for 1h. Cool to room temperature, filter, the filter cake is washed with methanol, and the filtrate is evaporated to remove the solvent to obtain a residue, which is obtained by H 2 O and CH 2 Cl 2 The mixed solvent (60mL, H 2 O and CH 2 Cl 2 The volume ratio is 1:1) Shake well to separate the liquid, and use CH for the water phase 2 Cl 2 (2 × 30mL) extraction; the combined organic phases were washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated to obtain a residue; the residue was separated by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =15:1 to3:1), to obtain compound 3 (0.40g, 89.5%, white solid).
[0045] Yield: 0.40g, 89.5%, white solid; R f =0.432(Petroleum eth...
Embodiment 3
[0046] Embodiment 3: the synthesis of compound 3
[0047] Repeat embodiment 2, difference is: replace methyl alcohol with THF, the mixed solvent that liquid separation is used changes into by H 2 The composition of O and EtOAc was 1:2; the reaction was carried out at 10°C, the reaction time was controlled at 10 h, and the rest remained unchanged. Finally a white solid (0.34 g, 75.8%) was obtained.
[0048] The obtained product was characterized by H NMR spectroscopy and identified as compound 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com