Dampproof effervescent bath ball and preparation method thereof
A technology of bath ball and alkali source, applied in the field of daily chemical products, can solve the problems of affecting the effect of bath ball, shortening the shelf life of storage, and failure of bath ball due to moisture, so as to ensure the quality of self-reaction, increase the sense of experience, and prolong the storage period.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
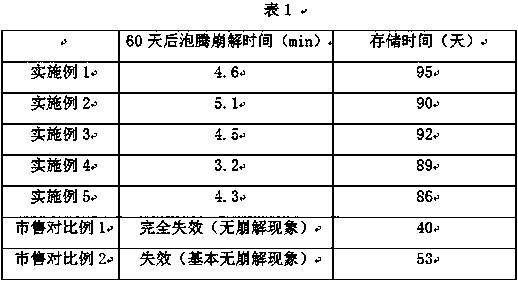
Examples
Embodiment 1
[0023] Alkali source pre-treatment: first dissolve 0.05 parts of water-soluble pigment to obtain colored water; then mix and stir the colored water with 30 parts of sodium carbonate until it is colored; finally, dry the colored sodium carbonate at 45°C for 15 minutes, and Pass through a 80-mesh sieve and set aside.
[0024] S1: Dissolve 1 part of ethyl cellulose in absolute ethanol or water to make a coating solution with a mass concentration of 5%;
[0025] S2: Mix the coating solution with the treated sodium carbonate for coating. After coating, dry at 45° C. for 15 minutes, and pass through an 80-mesh sieve to obtain intermediate product A;
[0026] S3: Mix intermediate product A with 5 parts of maltodextrin, 20 parts of adipic acid, 0.5 part of isopropanol, 0.01 part of isopropyl palmitate and 0.01 part of essence to obtain intermediate product B;
[0027] S4: The intermediate product B is pressed and molded, and dehumidified and dried to obtain a moisture-proof effervesc...
Embodiment 2
[0029] Alkali source pretreatment: first dissolve 0.3 parts of water-soluble pigment to obtain colored water; then mix and stir the colored water with 15 parts of potassium carbonate and 15 parts of potassium bicarbonate until the color is colored; finally, the colored potassium carbonate, carbonate Potassium hydrogen was dried at 50°C for 20 minutes, and passed through an 80-mesh sieve for use.
[0030] S1: Dissolve 6 parts of glyceryl behenate in absolute ethanol or water to prepare a coating solution with a mass concentration of 13%;
[0031] S2: Mix the coating solution with the treated potassium carbonate and potassium bicarbonate for coating. After coating, dry at 50° C. for 20 minutes and pass through an 80-mesh sieve to obtain an intermediate product A;
[0032] S3: Mix intermediate product A with 13 parts of sodium glutamate, 35 parts of citric acid, 1.8 parts of 95% ethanol, 0.1 part of isopropyl palmitate and 0.3 part of essence to obtain intermediate product B;
...
Embodiment 3
[0035] Alkali source pretreatment: first dissolve 0.1 part of water-soluble pigment to obtain colored water; then mix and stir the colored water with 60 parts of sodium bicarbonate until the color is colored; finally, dry the colored sodium bicarbonate at 55°C for 25 minutes , and pass through an 80-mesh sieve for later use.
[0036] S1: Dissolve 10 parts of methylcellulose in absolute ethanol or water to make a coating solution with a mass concentration of 20%;
[0037] S2: Mix the coating solution with 50 parts of fumaric acid for coating. After coating, dry at 55° C. for 25 minutes and pass through an 80-mesh sieve to obtain intermediate product A;
[0038] S3: Mix intermediate product A with 20 parts of filler, 50 parts of treated sodium bicarbonate, 0.3 part of polyethylene glycol 4000, 0.2 part of hydrogenated vegetable oil and 0.05 part of essence to obtain intermediate product B;
[0039] S4: The intermediate product B is pressed and molded, and dehumidified and dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com