Inosine injection and preparation method thereof
A technique for inosine injection and injection, which is applied in the field of inosine injection and its preparation, can solve problems such as unstable properties of inosine injection, and achieve the effects of avoiding adverse effects, stable performance, and avoiding yellowing of color and luster.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
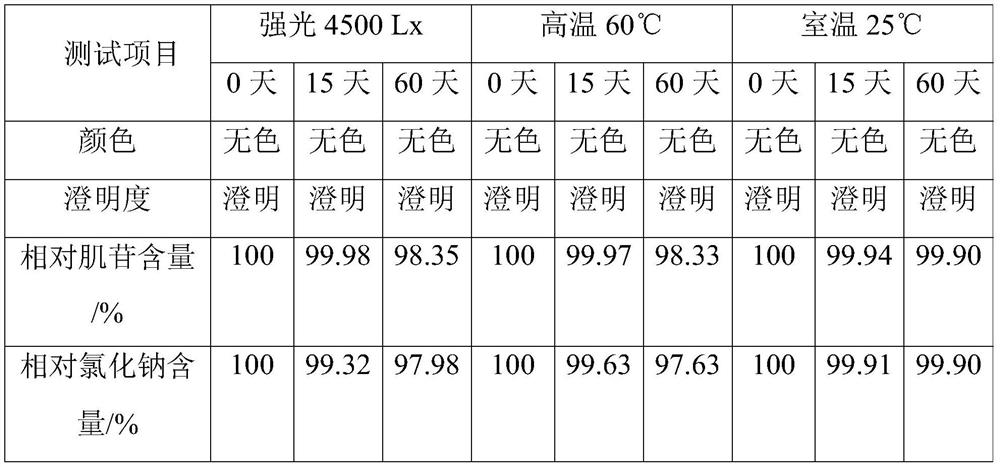
[0040] This example is used to illustrate the inosine injection of the present invention and its preparation method.
[0041] Accurately take the raw materials and auxiliary materials of each formulation amount with 1000L injection as the unit: inosine 5.9kg, sodium chloride 9.2kg, glycine 1kg, sodium benzoate 1.5kg, prepare this inosine injection by the following method:
[0042] (1) prepare the sodium hydroxide aqueous solution that pH value is 9.0;
[0043] (2) Add inosine to the aqueous sodium hydroxide solution, heat and stir until the inosine is dissolved, and obtain an aqueous solution of inosine;
[0044] (3) add sodium chloride after the inosine aqueous solution is incubated at 75° C. for 40 min, and then add glucosine and sodium benzoate to dissolve after the sodium chloride dissolves;
[0045] (4) Add 1 kg of activated carbon to the above solution, filter after stirring, add water for injection to dilute to 960L;
[0046] (5) Adjust the pH of the solution obtained...
Embodiment 2
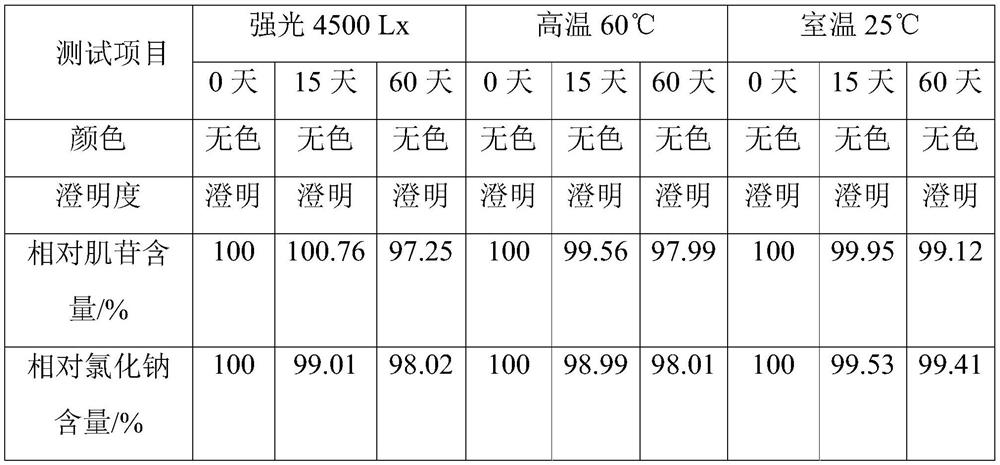
[0051] This example is used to illustrate the inosine injection of the present invention and its preparation method.
[0052] Accurately weigh the raw materials and auxiliary materials of each formulation amount in units of 1000L injection: inosine 6.1kg, sodium chloride 8.8kg, glycine 1.2kg, sodium benzoate 0.5kg. Prepare this inosine injection according to the following method:
[0053] (1) prepare the sodium hydroxide aqueous solution that pH value is 9.5;
[0054] (2) Add inosine to the aqueous sodium hydroxide solution, heat and stir until the inosine is dissolved, and obtain an aqueous solution of inosine;
[0055] (3) add sodium chloride after the inosine aqueous solution is incubated at 85° C. for 20 minutes, and then add glucosine and sodium benzoate to dissolve after the sodium chloride dissolves;
[0056] (4) Add 1.2kg of activated carbon to the above solution, filter after stirring, add water for injection to dilute to 980L;
[0057] (5) Adjust the pH of the sol...
Embodiment 3
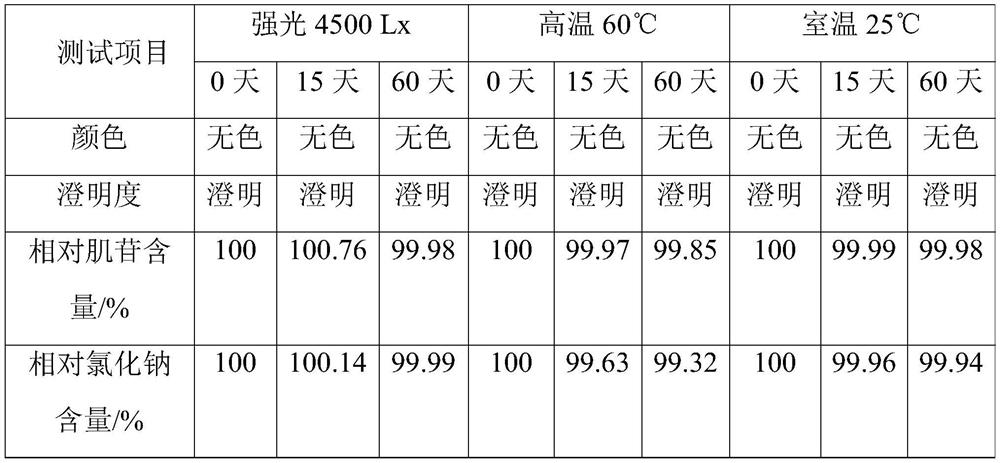
[0062] This example is used to illustrate the inosine injection of the present invention and its preparation method.
[0063] Accurately take by weighing the raw materials and auxiliary materials of each formulation amount with 1000L injection as the unit: inosine 6kg, sodium chloride 9kg, glycine 1.1kg, sodium benzoate 0.61kg. Prepare this inosine injection according to the following method:
[0064] (1) prepare the sodium hydroxide aqueous solution that pH value is 9.2;
[0065] (2) Add inosine to the aqueous sodium hydroxide solution, heat and stir until the inosine is dissolved, and obtain an aqueous solution of inosine;
[0066] (3) add sodium chloride after the inosine aqueous solution is incubated at 80° C. for 30 minutes, and then add glucosine and sodium benzoate to dissolve after the sodium chloride dissolves;
[0067] (4) Add 1.5kg of activated carbon to the above solution, filter after stirring, add water for injection to dilute, and dilute to 970L;
[0068] (5)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com