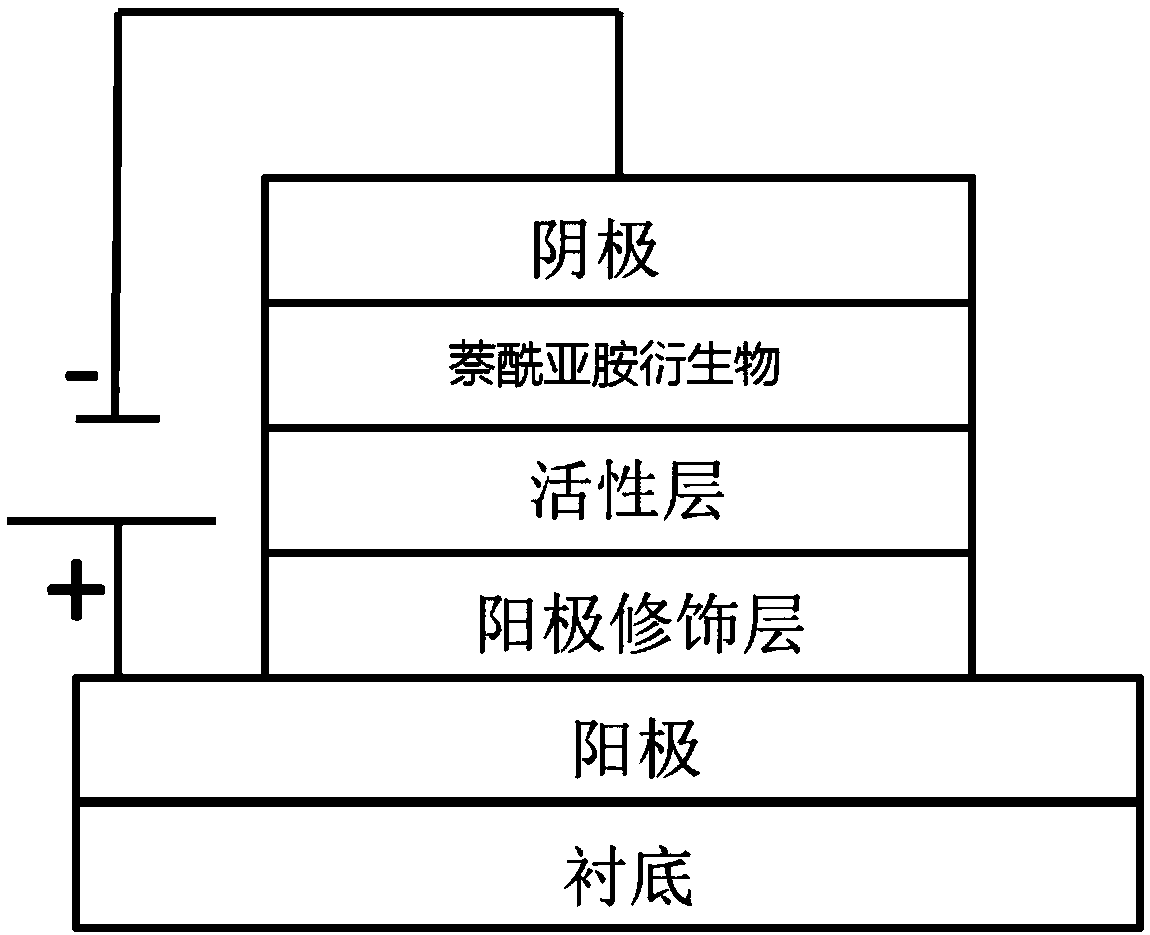
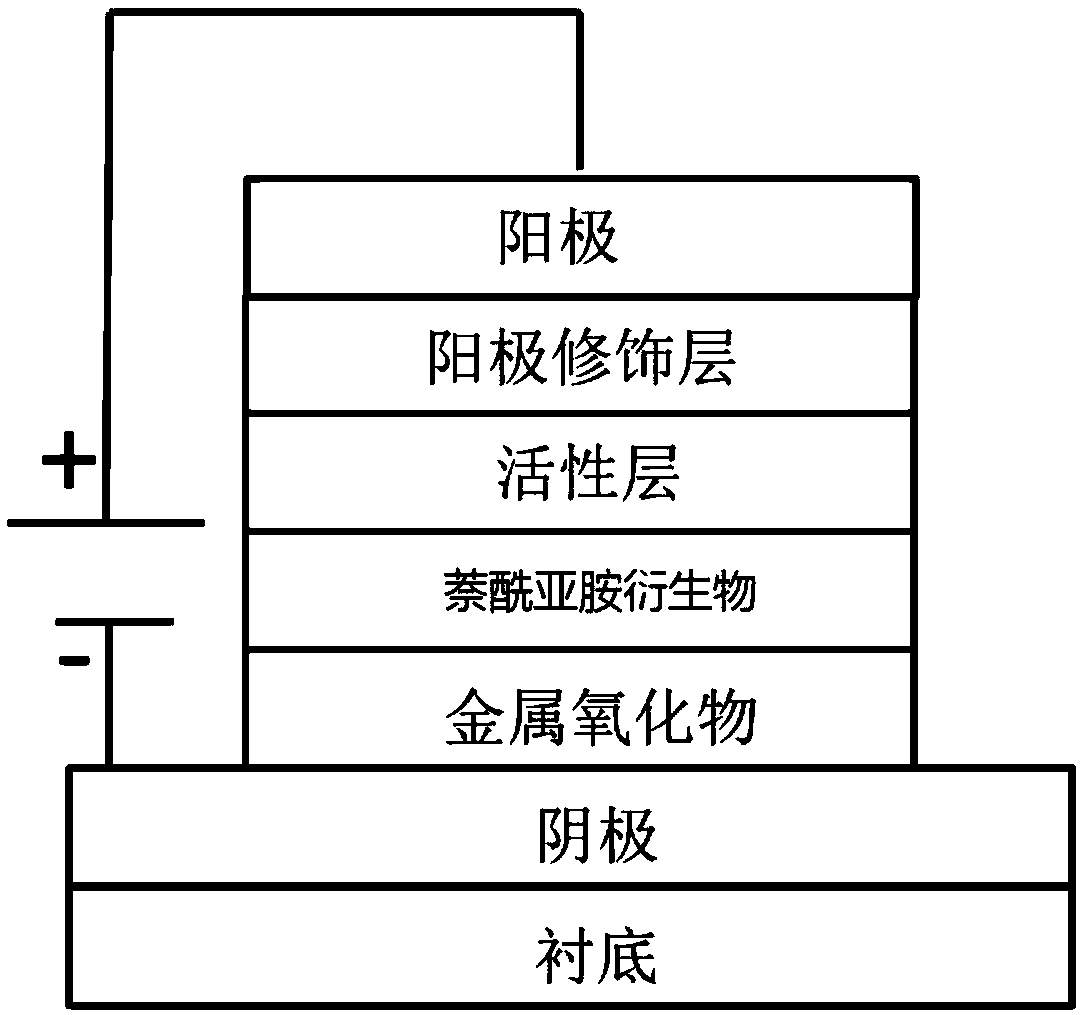
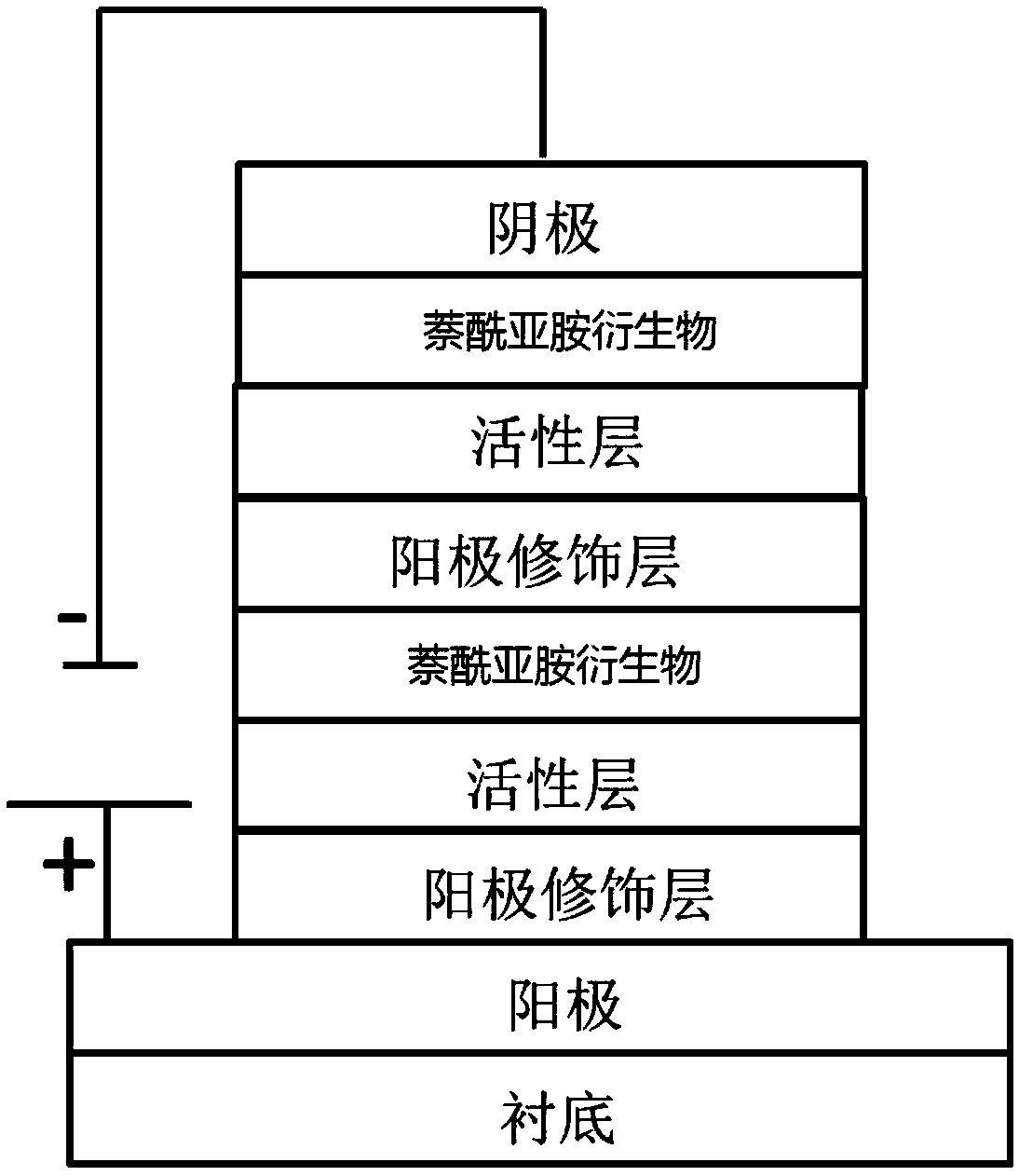
Naphthalimides derivatives and solar cell
A solar cell and naphthalimide technology, which is applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of uneconomical preparation methods and unsuitable low-temperature preparation processes, and achieve improved electron transport performance, excellent solubility, and reduced The effect of transmission loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Nitrogen, nitrogen-dimethylaminopropylamine-4-bromo-1,8-naphthalene dicarboxamide
[0054] 4-Bromo-1,8-naphthalic anhydride (5 g, 18 mmol) was suspended in 100 ml of ethanol, and added to reflux under nitrogen protection. 3-Dimethylaminopropylamine (2.21 g, 21.6 mmol) was added to the above reaction solution at one time, and reacted under reflux for 6 hours. The addition was stopped and cooled to room temperature, and the ethanol in the reaction solution was spin-dried to dry the solvent under reduced pressure to obtain a crude product. Then, the crude product was recrystallized in ethanol to obtain 4.88 g of the final yellow crystalline product. Its chemical reaction equation is as follows:
[0055]
Embodiment 2
[0056] Embodiment 2: Naphthalimide derivative 1
[0057] Under nitrogen protection, nitrogen, nitrogen-dimethylaminopropylamine-4-bromo-1,8-naphthalene dicarboxamide (1 g, 2.77 mmol) was mixed with trans-1,2-bis(tributyltin)ethylene ( 0.83 g, 1.38 mmol) was dissolved in 50 ml of tetrahydrofuran, and tetrahexatriphenylphosphine palladium (32 mg) was added, heated to reflux, and reacted for 24 hours. After cooling to room temperature, the tetrahydrofuran solvent was spin-dried under reduced pressure, and the crude product was recrystallized three times through tetrahydrofuran to obtain 512 mg of a yellow product. Its chemical reaction equation is as follows:
[0058]
Embodiment 3
[0059] Example 3: 6,6'-vinyl-bis(1,8-naphthalic anhydride)
[0060] Under nitrogen protection, 4-bromo-1,8-naphthalic anhydride (3 g, 10.8 mmol) and trans-1,2-bis(tributyltin)ethylene (3.27 g, 5.4 mmol) were dissolved in In 100 ml of tetrahydrofuran, tetrahexatriphenylphosphine palladium (62.4 mg) was added, heated to reflux, and reacted for 24 hours. After cooling to room temperature, the tetrahydrofuran solvent was spin-dried under reduced pressure. The crude product was purified by column chromatography with dichloromethane and recrystallized with chloroform to obtain 1.63 g of an off-white product. Its chemical reaction equation is as follows:
[0061]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com