Dihelicene functional molecular material of X-type heterocyclic perylene aromatic hydrocarbon, as well as preparation and application thereof
A technology of functional molecules and double helicenes, which is applied in the field of optoelectronic molecular materials, can solve the problem of not having both optical performance and transmission performance, and achieve the effect of broad development space.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
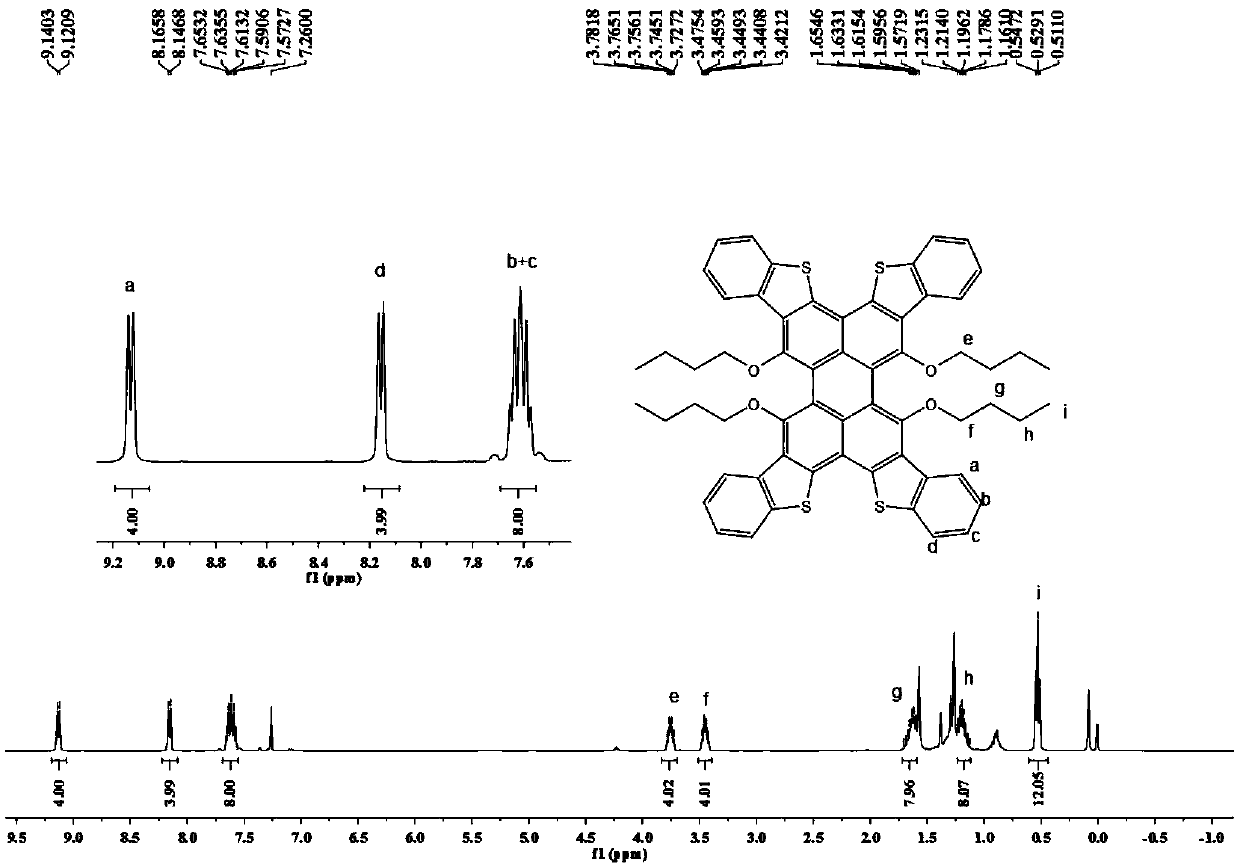
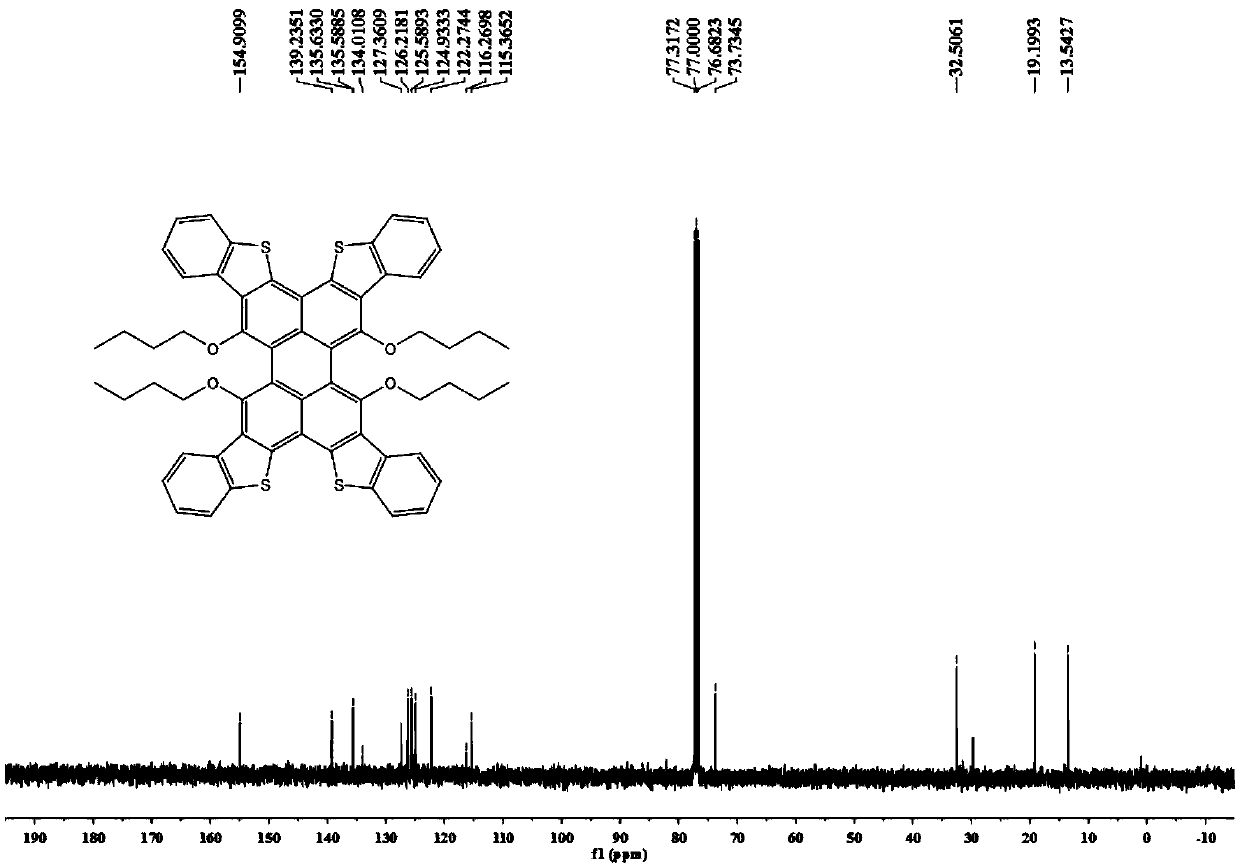
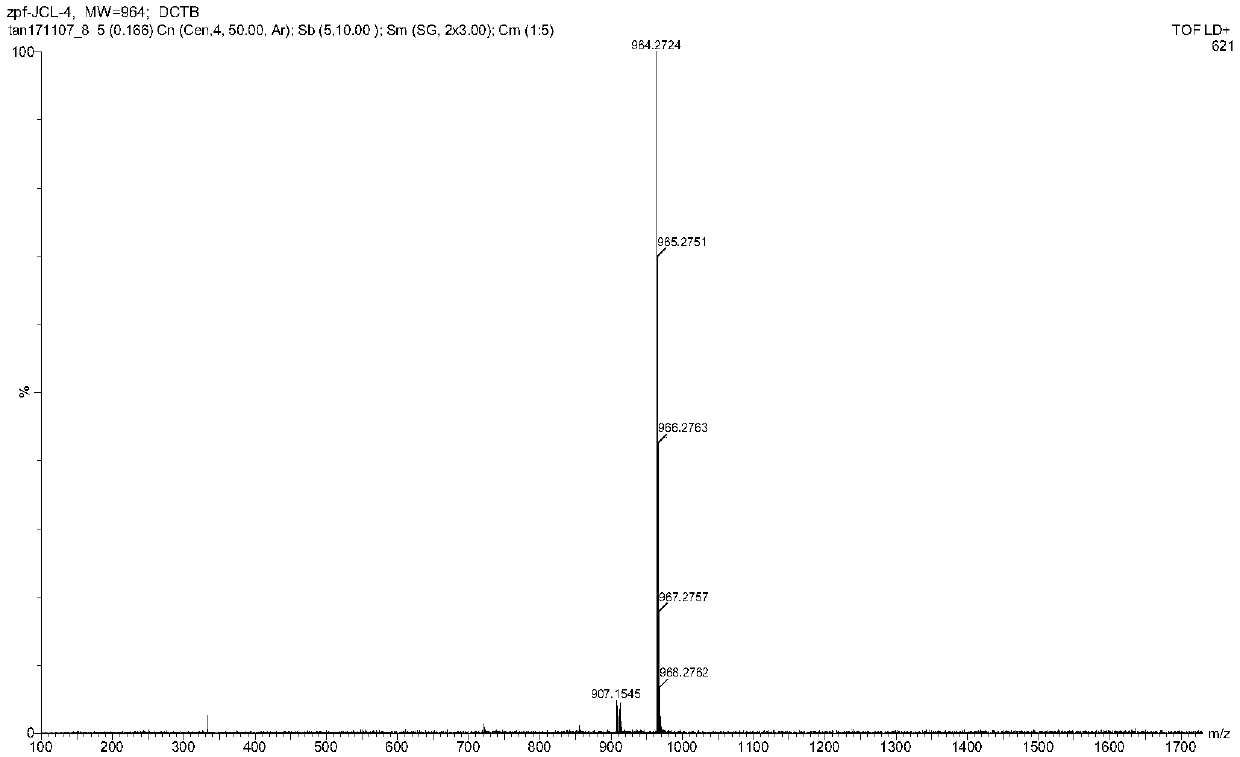
[0078] Synthesis of formula 1-A-1:
[0079] Add compound Per-4Br (in formula 3-A, R1~R4 is n-butoxy group) in dioxane, add 2-methylthiophenylboronic acid (1.2 equivalents), Pd ( dppf)Cl 2 (0.02 equivalent) and the reaction solvent (a mixed solvent of dioxane and water with a volume ratio of 5:1) carried out the Suzuki reaction at 85°C (±5°C), and the reaction was monitored by thin-layer chromatography. After the reaction Purification of the residue by silica gel chromatography, or by recrystallization, affords the intermediate product of Equation 1.
[0080] Add the product obtained above, chloroform, acetic acid and propylene oxide into an eggplant-shaped bottle, slowly add iodine solution (10 equivalents) dropwise after cooling to zero degrees Celsius, and then heat the reactant to 55°C (±5°C), and react For several hours, the reaction was monitored by thin-layer chromatography. After the reaction, the reaction was quenched with sodium bicarbonate, and the post-treatment w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com