Preparation method of 1-indanone and derivative thereof
A kind of derivative, indanone technology, applied in the field of preparation of 1-indanone and its derivatives, can solve the problems of low solid acid yield, a large amount of waste acid and the like, and achieves convenient recycling, convenient operation and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A preparation method of 1-indanone and derivatives thereof, the method comprising:
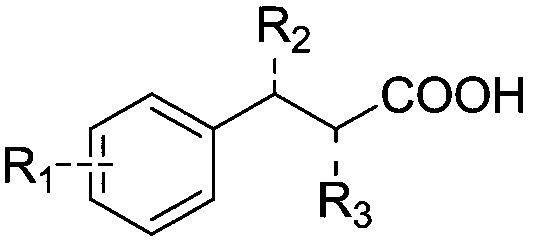
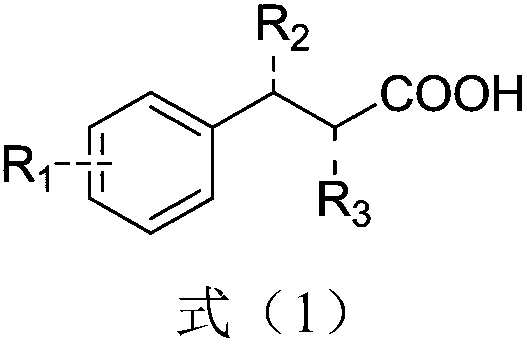
[0023] React phenylpropionic acid and its derivatives, heteropolyacids, and phase-transfer catalysts with the structure shown in formula (1) in an aprotic, non-polar organic solvent at a reaction temperature of 60-150°C, and undergo a dehydration acylation reaction , the reaction solvent is separated from the heteropolyacid and the phase transfer catalyst, the reaction solvent is concentrated to separate out 1-indanone and its derivatives, and the separated heteropolyacid and phase transfer catalyst are recycled, which can continue to catalyze the next pot reaction without deactivation .
[0024]
[0025] Among them, R 1 , R 2 and R 3 are each independently H, alkyl or alkoxy.
[0026] Further, the above R 1 , R 2 and R 3 are each independently H, Me or OMe.
[0027] The above-mentioned aprotic and non-polar organic solvents include any one or two or more of petroleum ether (...
Embodiment 1
[0035] Dissolve 0.6g (4mmol) of phenylpropionic acid in 15mL of cyclohexane, add 1.15g (0.4mmol) of phosphotungstic acid and 0.064g (0.2mmol) of tetrabutylammonium bromide, heat and stir at 90°C under heterogeneous conditions, Reflux for 2 hours, filter with suction, remove cyclohexane, the residue solidified and precipitated white crystals of 1-indanone, the yield was higher than 96%; m.p.41-43 ° C; 1H-NMR (CDCl 3 , 400MHz): δ7.31-7.35(m, 2H), 7.21-7.25(m, 2H), 2.99(t, J=6.0Hz, 2H); 2.71(t, J=6.0Hz, 2H).
Embodiment 2
[0037] Dissolve 0.66g (4mmol) of 4-methylphenylpropionic acid in 15mL petroleum ether, add 1.15g (0.4mmol) of phosphotungstic acid and 0.045g (0.2mmol) of benzyltriethylammonium chloride, under heterogeneous conditions Heated and stirred at 120°C and refluxed for 2 hours, filtered with suction to remove petroleum ether, the residue solidified and precipitated white crystals of 6-methylindanone, the yield was higher than 95%; m.p.62-64°C; 1H-NMR (CDCl 3 ,300MHz):δ7.56(s,1H),7.35-7.42(m,2H),3.10(t,J=6.0Hz,2H); 2.69(t,J=6.0Hz,2H),2.40(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com