Coumarin compound and extracting method thereof
A technology of coumarins and extraction methods, which is applied in the fields of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., and can solve the problems that the anticancer effect has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
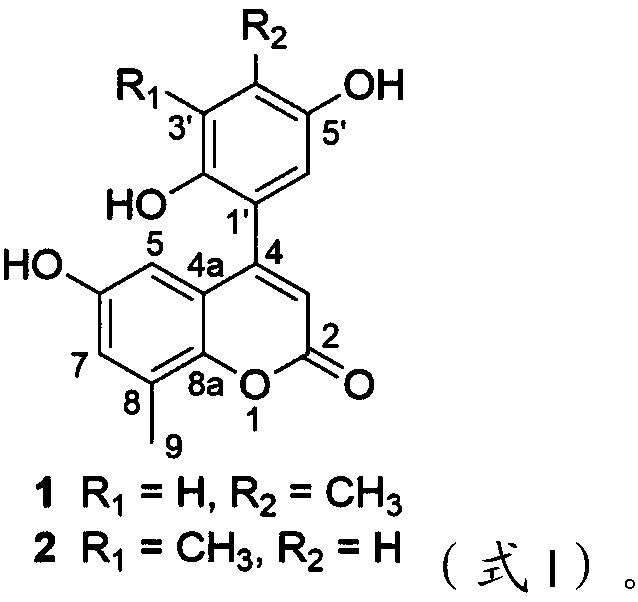
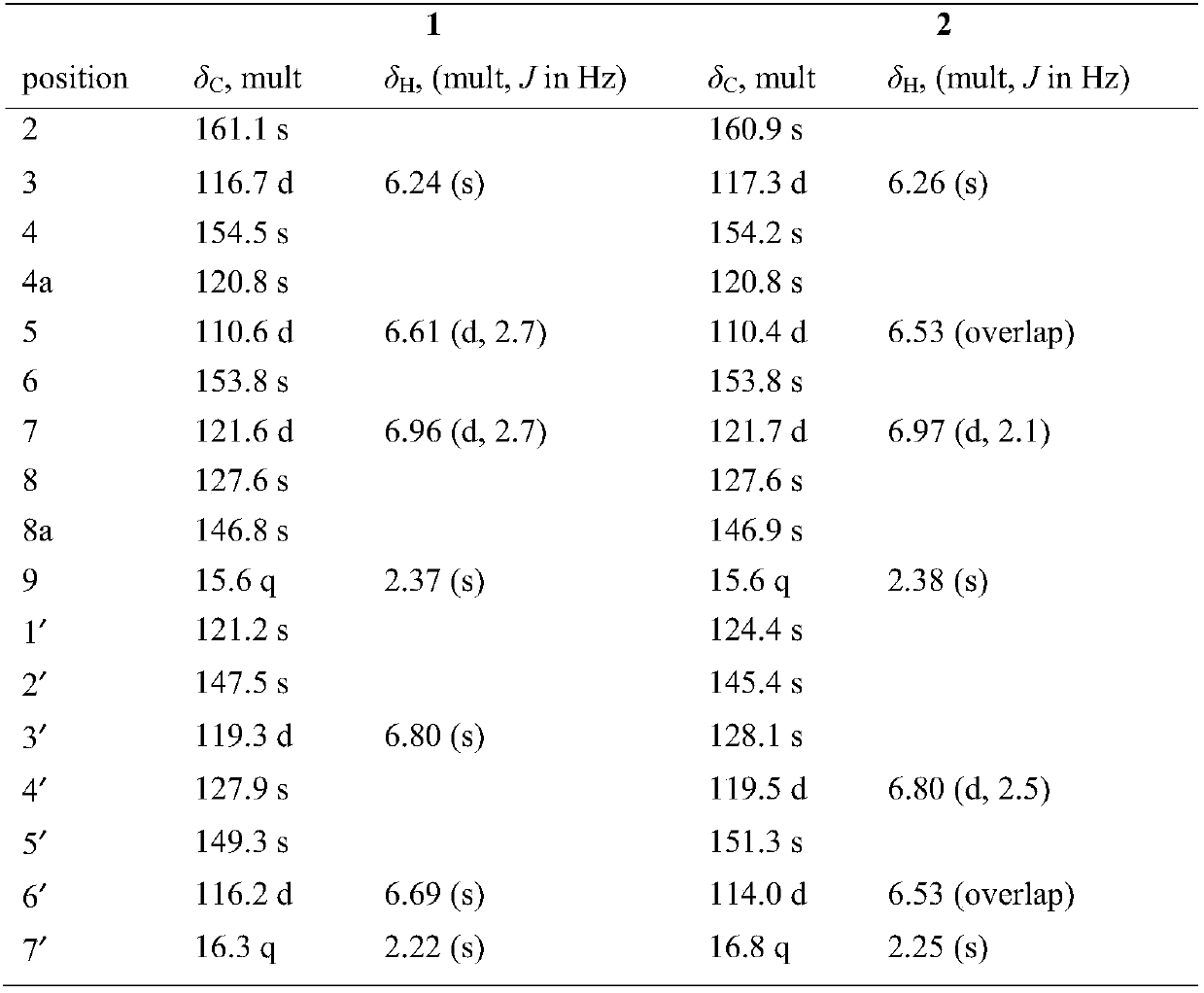
[0025] Example 1 Extraction, separation and structural identification of the compound of the present invention
[0026] Japanese Pipa A 50kg, pulverized, cold soaked and extracted with 70% ethanol for 3 times, each time for 48h, stirred from time to time, filtered, and recovered ethanol under reduced pressure to obtain 4kg of extract, suspended in acid water, adjusted to pH 1-2, washed with ethyl acetate The ester was extracted 4 times with equal volume to obtain 1 kg of non-alkaloid fraction. The remaining acidic aqueous layer was adjusted to neutral pH with alkali, extracted 4 times with equal volume of ethyl acetate to obtain 300 g of alkaloid fraction. The non-alkaloid fraction (1kg) was chromatographed on MCI gel CHP 20P column, methanol / water (10:90,20:80,30:70,40:60,50:50,60:40,70:30,80: 20, 90:10, 100:0) gradient elution, and TLC detection to combine the same fractions to obtain a total of 6 fractions from Fractions A-F. Fr.E (75g) was separated by Sephadex LH-20 col...
Embodiment 2
[0032] Embodiment 2 compound antitumor activity (A549, K562 and Huh-7) test method
[0033] The method for detecting the antitumor activity of compounds (A549, K562 and Huh-7) in vitro using CCK-8 reagent. Specific steps are as follows:
[0034] Various cancer cell lines were plated on a 384-well plate with a volume of 50 μL / well at an appropriate cell concentration, and cultured overnight at 37°C.
[0035] Compound dilution: first dissolve the compound into 10mM stock solution with DMSO, and dilute it into 10 concentration gradients with DMSO 3-fold gradient to make 1000×compound serial concentration stock solution, and then transfer to compound transfer instrument adapted to 384PP plate for later use.
[0036] Using the compound transfer instrument Liquid Handler Echo520, the compounds at 1000× serial concentrations were transferred to the corresponding wells of the cancer cell 384-well plate, 50 nL per well, and 50 nL DMSO was added to the blank control wells. Mix gently,...
Embodiment 3
[0043] The compound in Example 1 can be made into injection after adding injection solvent according to the conventional method, finely filtering, potting and sterilizing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com