Ev-mediated delivery of binding protein-small molecule conjugates
A technology that combines proteins and small molecules, applied in the field of protein-drug conjugates, can solve the problems of EV loading with less small molecules and practical therapeutic applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Apomorphine-loaded EVs for Parkinson's disease
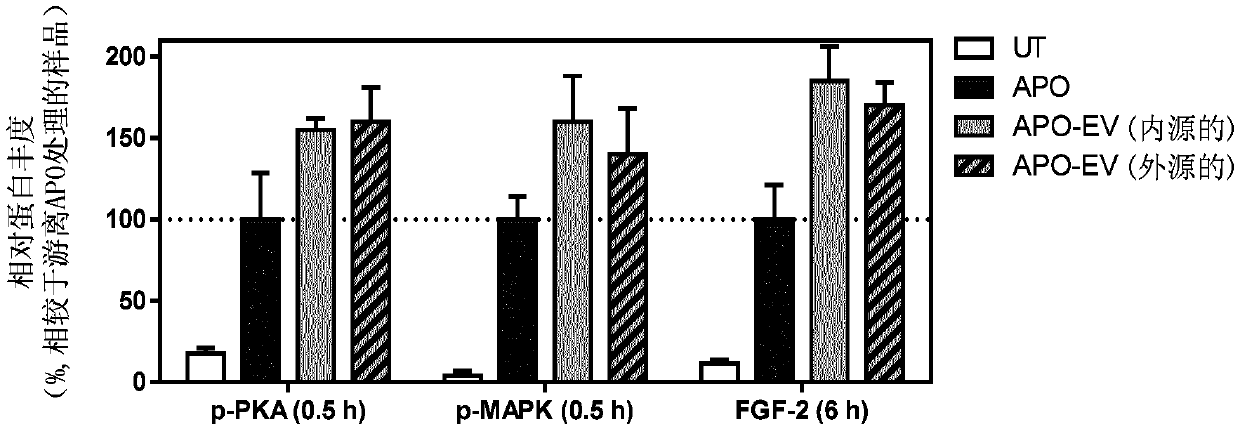
[0064] MSCs endogenously expressing dopamine receptor D2 and MSCs genetically engineered to express a fusion protein comprising EV protein CD63 and dopamine receptor D2 were cultured in MSCGM growth medium. To endogenously load MSC-EVs with apomorphine (APO), a drug used to treat Parkinson's disease, MSC medium was replaced with Opti-EM medium and incubated with 2 μM APO for 12 hours. Thereafter, conditioned medium was collected and APO-EVs were isolated by tangential flow filtration and size exclusion chromatography. To exogenously load MSC-EVs with APO, EVs from untreated MSCs were isolated as above, then incubated with 2 μM APO for 2 h, and re-isolated to remove APO molecules not loaded into EVs. APO loading to EVs is facilitated through the interaction of its dopamine receptor D2 on the EV surface, which results in a "precharge" of the dopamine receptors capable of signaling.
[0065] The activity of APO-...
Embodiment 2
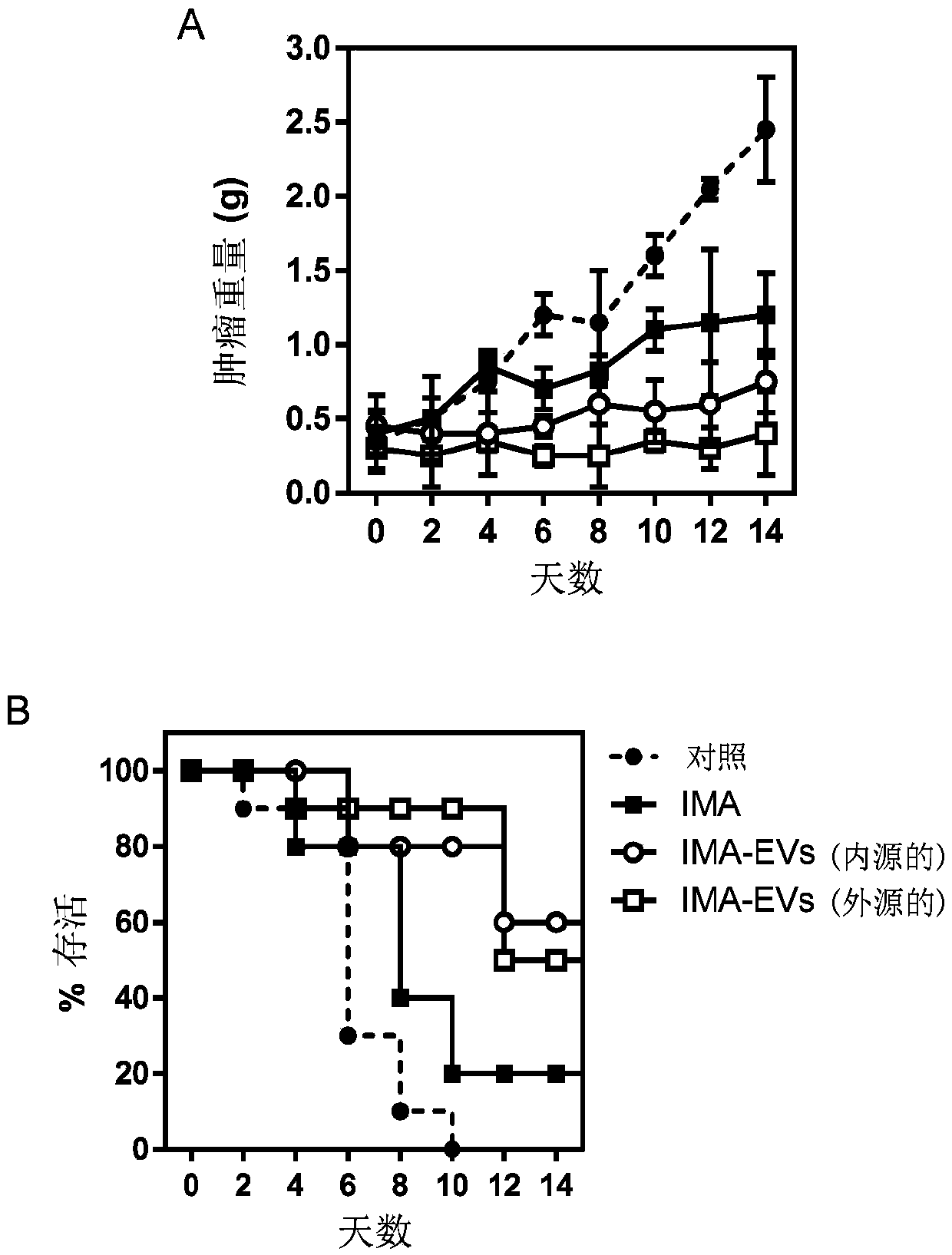
[0066] Example 2: Loading of Imatinib into EVs for Cancer Therapy
[0067] MSCs endogenously expressing ABL1 receptor tyrosine kinase and MSCs genetically engineered to express a fusion protein of the EV protein syntenin and ABL1 receptor tyrosine kinase were cultured in MSCGM growth medium. To endogenously load MSC-EVs with imatinib (IMA), MSC medium was replaced with Opti-MEM medium and incubated with 2 mg IMA. Thereafter, conditioned medium was collected and IMA-EVs were isolated by tangential flow filtration and size exclusion chromatography. To exogenously load MSC-EVs with IMA, EVs from untreated MSCs were isolated as described above, then incubated with 2 mg IMA for 2 h, and re-isolated to remove IMA molecules not loaded into EVs
[0068] IMA is a drug used in the treatment of chronic myelogenous leukemia (CML) because it binds to ABL1 and subsequently stabilizes the Bcr-Abl receptor tyrosine kinase complex, making IMA a potent inhibitor of the kinase. Loading of IMA ...
Embodiment 3
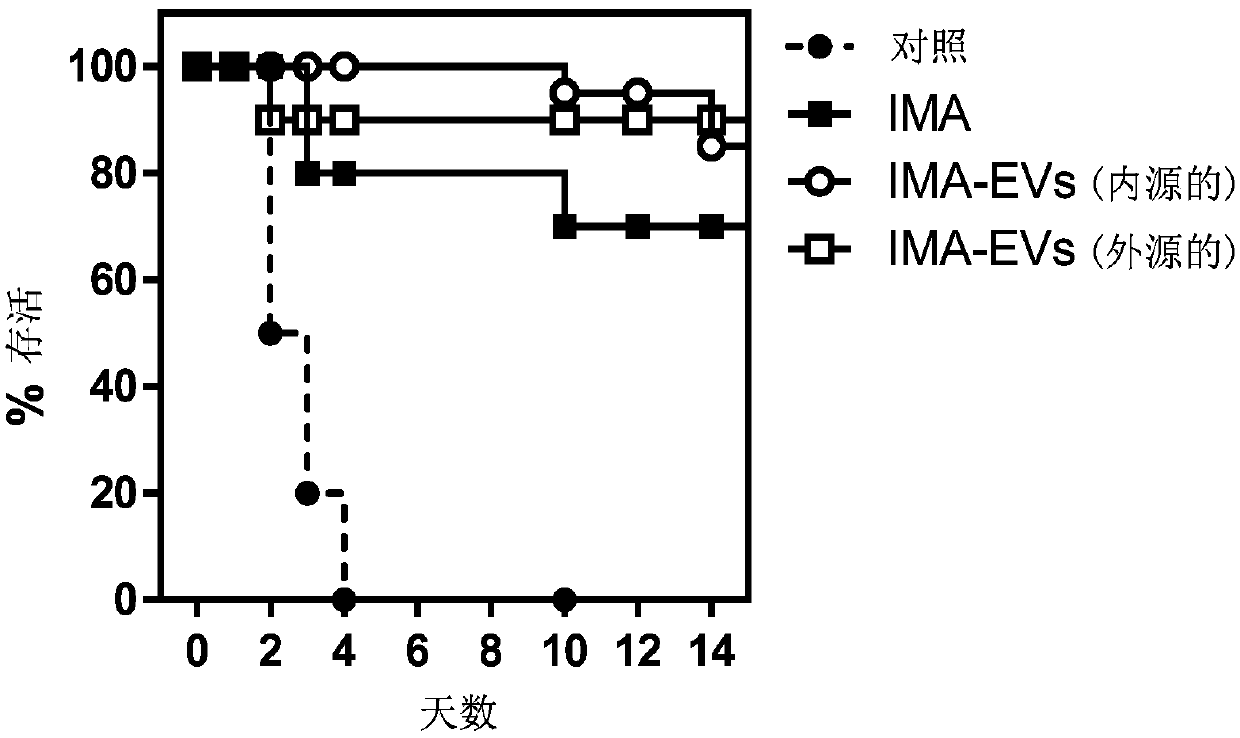
[0070] Example 3: Imatinib Loading to EVs for Prevention of Intravenous LPS-Induced Sepsis
[0071] Cell culture, EV loading and EV isolation were performed as in Example 2, but the activity of EV loaded IMA was tested in an intravenous LPS-induced acute sepsis model. LPS treatment is known to induce high levels of reactive oxygen species associated with septic shock and ARDS. Increased IMA function of catalase activity was associated with reduced DNA damage and decreased production of the pro-inflammatory cytokines TNF-α and IL-6.
[0072] Mice were sensitized to LPS by retro-orbital injection of 5 mg / kg LPS. IMA and EV-IMA treatments were initiated the day before daily administration with an equivalent amount of 200 mg / kg / day of IMA, and mouse survival was monitored.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com