Methods of measuring exosomes using intrinsic fluorescence
a technology of exosomes and fluorescence, applied in the direction of fluorescence/phosphorescence, material analysis through optical means, instruments, etc., can solve the problems of inability to accurately and rapidly measure the absolute number of extracellular vesicles, laborious and time-consuming current methods, and current ultra-centrifugation protocols are commercially unreproducibl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ion Exchange Chromatography Profiles of Purified Exosomes and Cell Culture Harvest at Different Excitation and Emmission Spectra
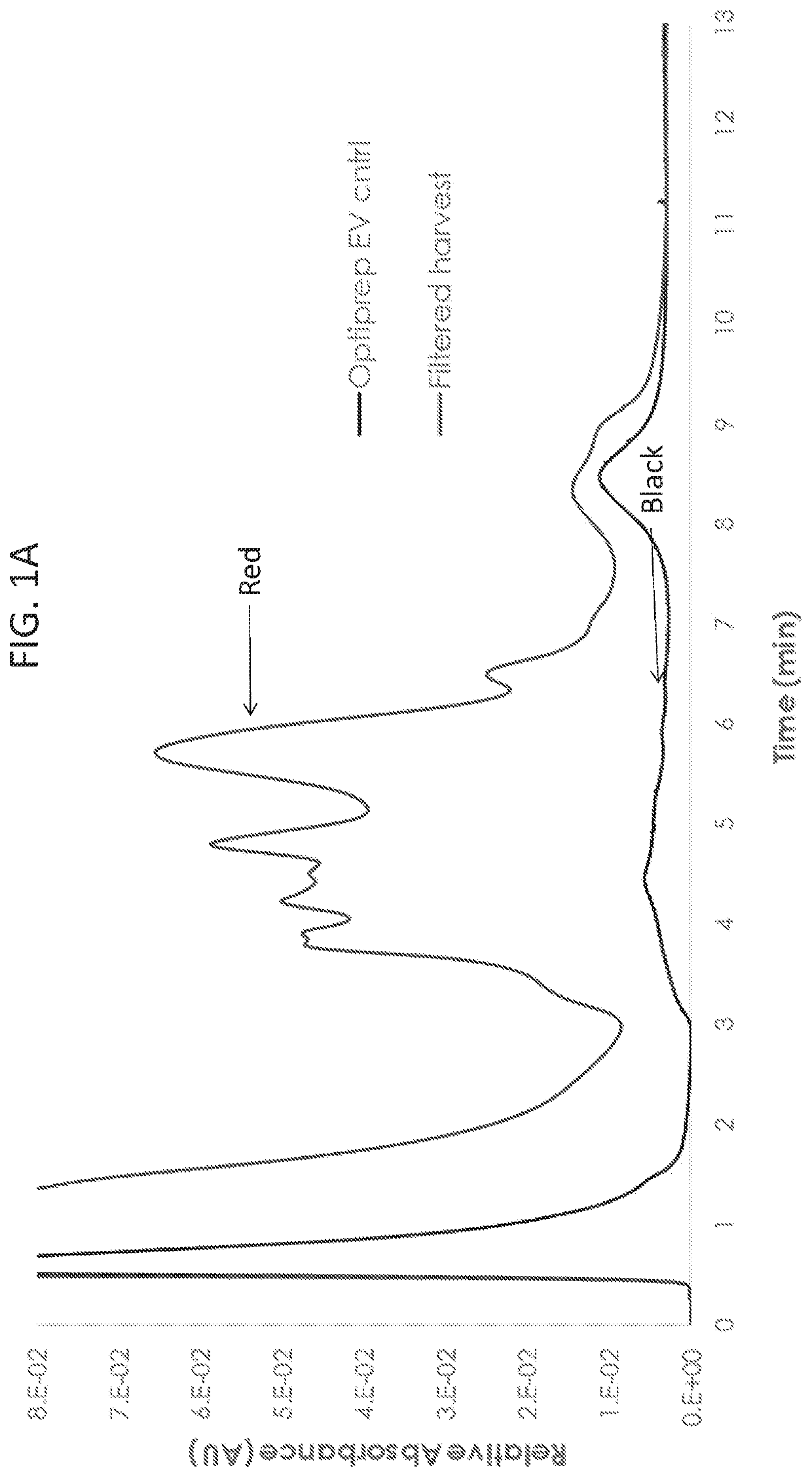
[0083]To understand fundamental properties of exosomes and develop analytical tools for studying exosomes, Optiprep™ purified exosome preparations and cell culture harvest were analyzed by strong anion exchange chromatography (AEX). Exosomes from HEK293SF cells grown in serum-free conditions were processed as described in the methods above. For cell culture harvest, HEK293SF cells were grown in serum-free medium, and 600 ml of supernatant were collected. Cell culture harvest was then centrifuged at 1600 g for 10 minutes and filtered through a 0.8 μm filter to remove cellular debris before chromatographic analysis. AEX was monitored at 210, 254, and 280 nm and was performed using a CIMac monolithic QA-1 mL column from BIA Separations. The Optiprep™ purified exosome stock concentration was measured to be 4×1012 particles / mL by nanoparticle tracking assay (NTA...
example 2
ng the Optimal Fluorescence Spectrum for Analyzing AEX-Purified Exosomes
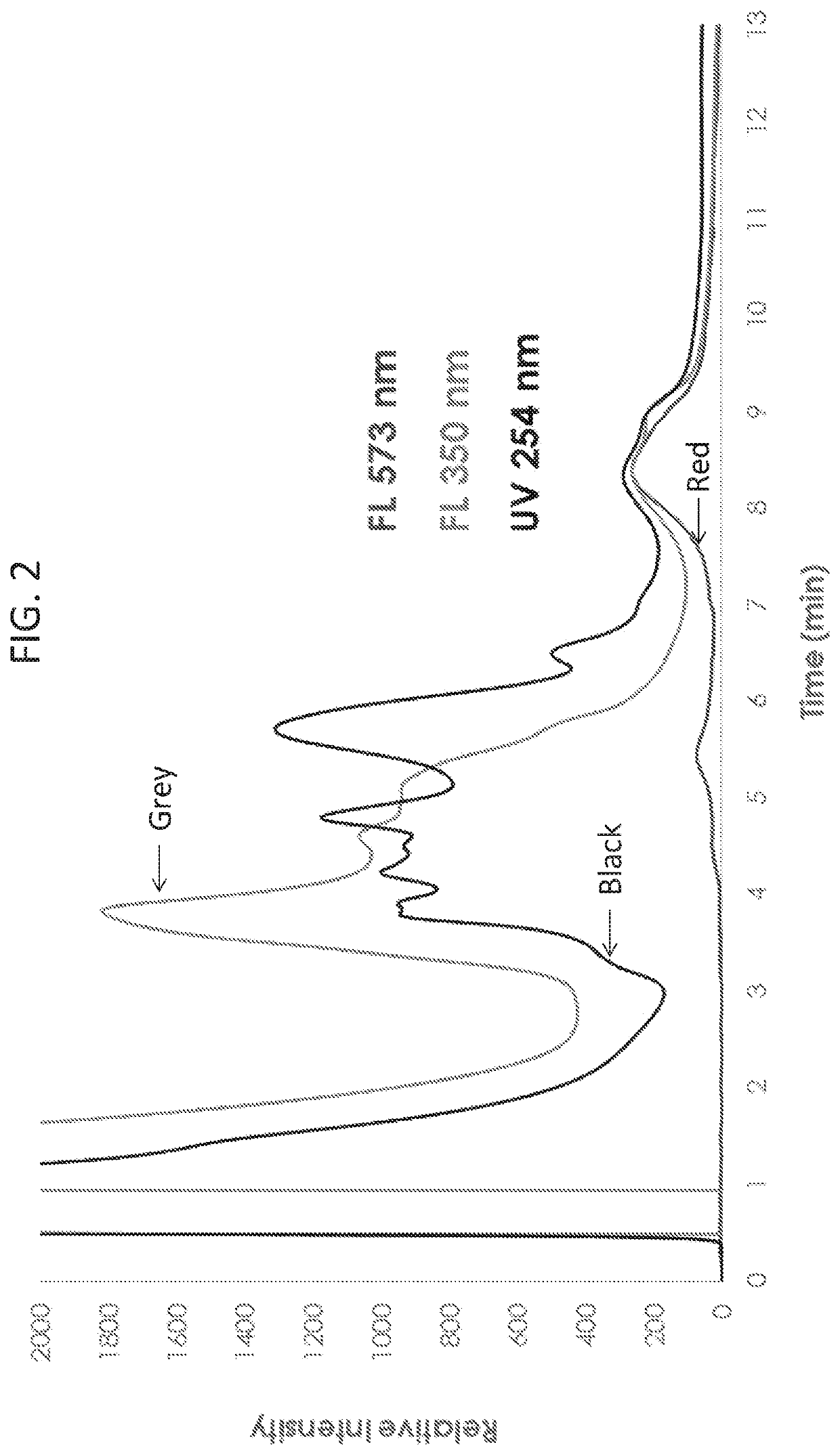
[0086]To optimize the excitation and emission wavelengths for the detection of exosomes, wavelength scanning on Optiprep™ purified exosomes was carried out. To determine the optimal excitation wavelength, Optiprep™ purified exosomes were analyzed by AEX, while the emission wavelength was held constant at 573 nm, and the excitation wavelength was scanned from 280 nm to 560 nm (FIG. 4A). To determine the optimal emission wavelength, Optiprep™ purified exosomes were analyzed by AEX, while the excitation wavelength was held constant at 556 nm, and the emission wavelength was scanned from 570 nm to 770 nm (FIG. 4B). These results indicate that the optimal wavelengths for detecting intrinsic fluorescence of exosomes is an excitation wavelength of 556 nm and emission wavelength of 573 nm.
example 3
Liposomes and Cell-Derived Exosomes Do Not Have the Same Fluorescence Spectra
[0087]To determine if the fluorescence profile determined in Example 1 was specific to cell-derived exosomes, synthetic liposomes were also analyzed by AEX at ex556 / em573. Synthetic clodronate liposome controls formulated in PBS were purchased. The liposomes were composed of a mixture of phosphatidylcholine and cholesterol and formulated to be between 0.15-3 μm in size. Before analysis, the liposomes were filtered through a 0.45 μm cellulose acetate filter to remove the larger sized liposomes. The resulting filtered liposomes were analyzed by NTA (FIG. 5A) and transmission electron microscopy (FIG. 5B) confirming that they are similar in size and shape to cell-derived exosomes (see FIG. 1B).
[0088]To confirm that the synthetic liposomes had similar size-dependent characteristics of cell-derived exosomes, both populations were analyzed by size-exclusion chromatography (SEC) and monitored by UV absorbance. As ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com