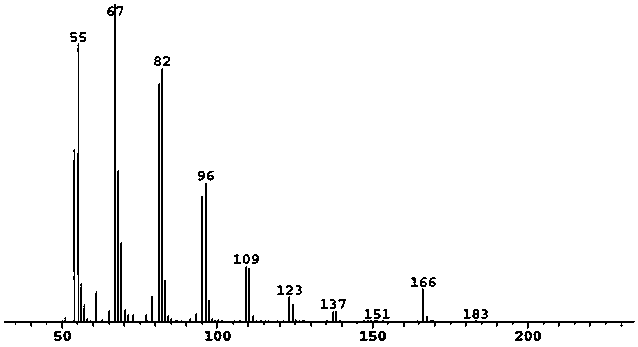
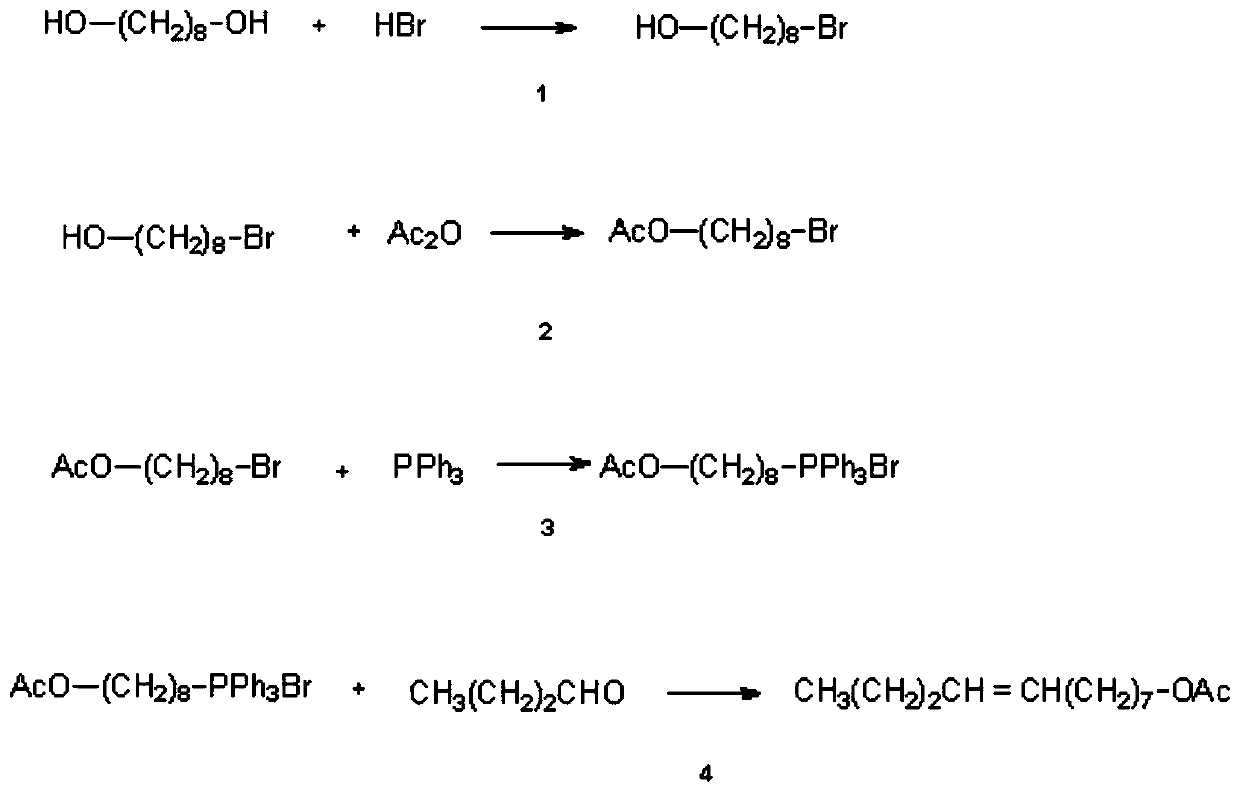
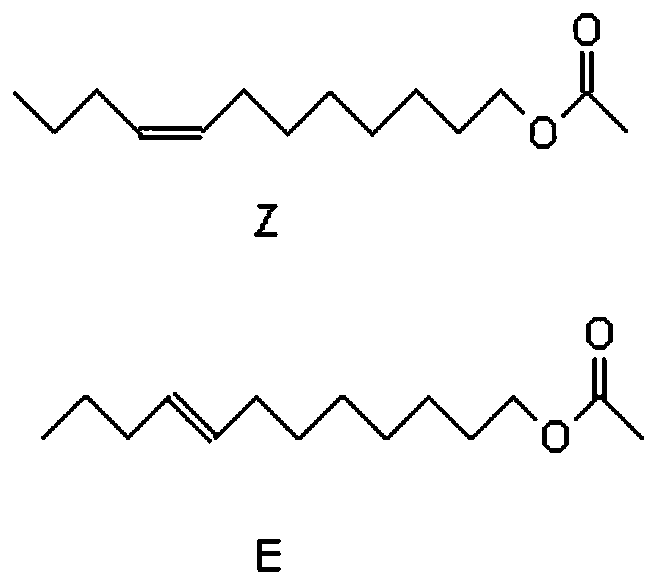
Synthesis method of (Z/E)-8-dodecen-1-alcohol acetate compound
A technology of alcohol acetate and dodecene, applied in the field of pesticides, can solve the problems of difficult post-processing, unfavorable large-scale production, and many impurities in the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) Synthesis of 8-bromooctanol
[0062] Put 7.3g (50mmol) of 1,8-octanediol and 10.0g of 40% hydrobromic acid (50mmol) into 150ml of toluene, heat and reflux for 8-20h, after the reaction is completed, neutralize, wash with water, and dry over anhydrous sodium sulfate , Distilled to obtain 9.9 g of 8-bromooctanol with a yield of 95%.
[0063] (2) Synthesis of 8-bromooctyl acetate
[0064] 10.4g (50mmol) 8-bromooctyl alcohol and 50mL pyridine, 5.3g (50mmol) acetic anhydride were reacted at room temperature for 24h. After the reaction was completed, the product was isolated and subjected to silica gel column chromatography to obtain 12.1g 8-bromooctyl alcohol acetate. rate 97.5%,
[0065] (3) Synthesis of ω-acetoxyoctyltriphenylphosphonium bromide salt
[0066] Put 12.50g (50mmol) of 8-bromooctyl acetate and 13.10g (50mmol) of triphenylphosphine into 150ml of benzene, heat and reflux for 20h, and when the reaction is complete, pour out the benzene layer and remove the...
Embodiment 2
[0070] (1) Synthesis of 8-bromooctanol
[0071] Put 7.3g (50mmol) of 1,8-octanediol and 10.0g of 40% hydrobromic acid (50mmol) into 120ml of toluene, heat and reflux for 8-20h, after the reaction is completed, neutralize, wash with water, and dry over anhydrous sodium sulfate , Distilled to obtain 9.6 g of 8-bromooctanol with a yield of 92.3%.
[0072] (2) Synthesis of 8-bromooctyl acetate
[0073] 10.4g (50mmol) 8-bromooctyl alcohol and 50mL pyridine, 5.3g (50mmol) acetic anhydride were reacted at room temperature for 20h. After the reaction was completed, the product was isolated and subjected to silica gel column chromatography to obtain 12.0g 8-bromooctyl alcohol acetate. Rate 96.0%,
[0074] (3) Synthesis of ω-acetoxyoctyltriphenylphosphonium bromide salt
[0075] Put 12.50 (50 mmol) of 8-bromooctyl acetate and 13.10 (50 mmol) of triphenylphosphine into 120 ml of benzene, heat and reflux for 15 hours, and when the reaction is complete, pour out the benzene layer and re...
Embodiment 3
[0079] (1) Synthesis of 8-bromooctanol
[0080] Put 7.3g (50mmol) of 1,8-octanediol and 10.0g of 40% hydrobromic acid (50mmol) into 120ml of toluene, heat and reflux for 8-20h, after the reaction is completed, neutralize, wash with water, dry over anhydrous sodium sulfate, After distillation, 9.8 g of 8-bromooctanol was obtained with a yield of 94.2%.
[0081] (2) Synthesis of 8-bromooctyl acetate
[0082] 10.4g (50mmol) 8-bromooctyl alcohol and 50mL pyridine, 5.3g (50mmol) acetic anhydride were reacted at room temperature for 24h. After the reaction was completed, the product was isolated and subjected to silica gel column chromatography to obtain 12.2g 8-bromooctyl alcohol acetate. rate 97.6%,
[0083] (3) Synthesis of ω-acetoxyoctyltriphenylphosphonium bromide salt
[0084] Put 12.50g (50mmol) of 8-bromooctyl acetate and 13.10g (50mmol) of triphenylphosphine into 120ml of benzene, heat and reflux for 15h, after the reaction is over, pour out the benzene layer, remove the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com