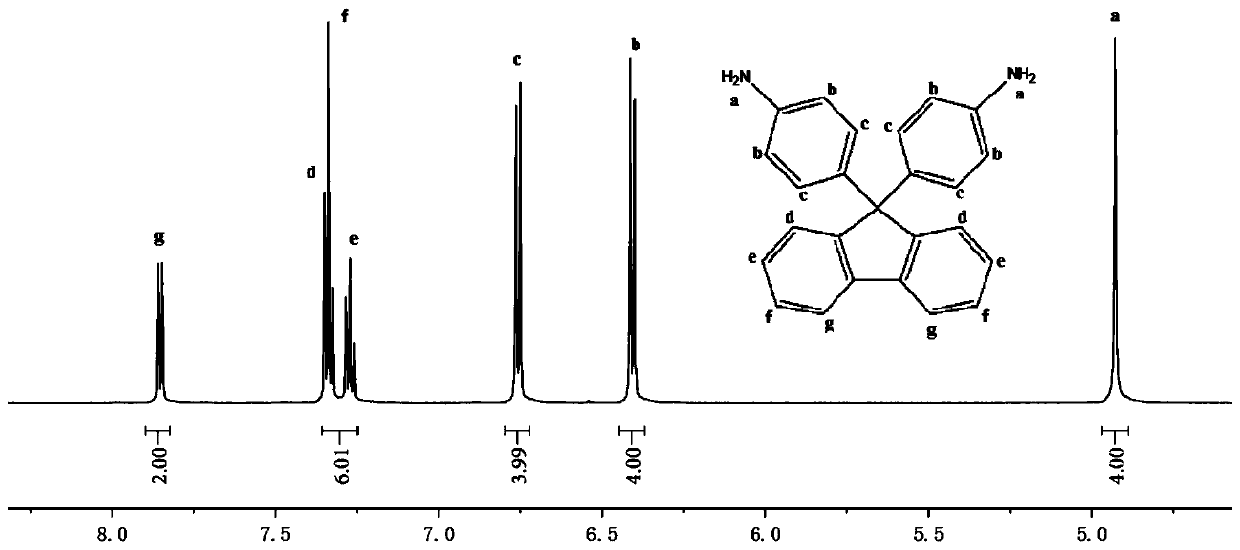
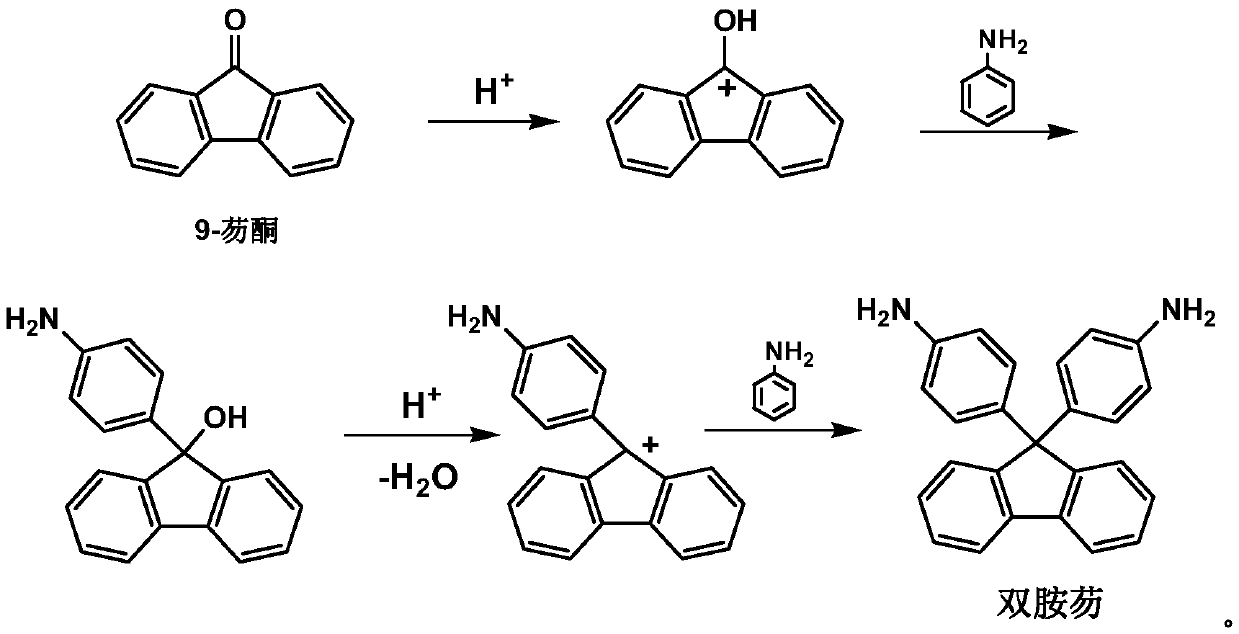
Preparation method of 9,9-bis(4-aminophenyl) fluorene
A technology of diamine fluorene and aniline, which is applied in the field of preparation of bisamine fluorene, can solve the problems of complicated post-processing, low product purity, difficult recovery and the like, and achieves simple post-processing, improved purity and yield, and reduced labor costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0039] Example 1
[0040] Step 1: Add 103g of aniline and 11g of hydrochloric acid to a 250ml three-necked flask equipped with a stirrer, thermometer and water separator. After stirring for 10 minutes, add 18g of 9-fluorenone and 50ml of toluene. The temperature will be refluxed for 6-10 hours, and the system will be separated. water;
[0041] Step 2: Cooling, sampling, thin-layer chromatography analysis, after there is no raw material 9-fluorenone, add 13g of 35% NaOH aqueous solution to the system, stir for 30min, heat and reflux, and separate the water in the system;
[0042] Step 3: Recover toluene: increase the temperature, return toluene to the water separator and collect; collect 48ml of toluene, and then extract 77g of aniline from the system under reduced pressure. The recovery rate of toluene and aniline reaches 92%;
[0043] Step 4: Add absolute ethanol to the system to disperse, filter and collect the filter cake, add the filter cake to 150ml of water and stir for 30 minut...
Example Embodiment
[0044] Example 2
[0045] Step 1: Add 515g of aniline and 55g of hydrochloric acid to a 1000ml three-necked flask equipped with a stirrer, a thermometer and a water separator. After stirring for 15 minutes, add 90g of 9-fluorenone and 100ml of toluene. The temperature will be refluxed for 10 hours to separate the water in the system;
[0046] Step 2: Cooling, sampling, thin layer chromatography analysis, after no raw material 9-fluorenone, add 65g of 35% NaOH aqueous solution to the system, stir for 30min, heat up and reflux, and separate the water in the system;
[0047] Step 3: Recover toluene: increase the temperature, return toluene to the water separator and collect; collect 95ml of toluene, and then extract 392g of aniline from the system under reduced pressure. The recovery rate of toluene and aniline is 93%;
[0048] Step 4: Add absolute ethanol to the system for dispersion, filter and collect the filter cake, add the filter cake to 150ml of water and stir for 30 minutes to was...
Example Embodiment
[0049] Example 3
[0050] Step 1: Add 465g of aniline and 75g of hydrochloric acid to a 1000ml three-necked flask equipped with a stirrer, a thermometer and a water separator. After stirring for 15 minutes, add 90g of 9-fluorenone and 100ml of toluene, and reflux for 10 hours to separate the water in the system;
[0051] Step 2: Cooling, sampling, thin-layer chromatography analysis, after there is no raw material 9-fluorenone, add 80g of 40% NaOH aqueous solution to the system, stir for 30min, heat and reflux, and separate the water in the system;
[0052] Step 3: Recover toluene: increase the temperature, return toluene to the water separator and collect; collect 95ml of toluene, and then extract 353g of aniline from the system under reduced pressure. The recovery rate of toluene and aniline is 95%;
[0053] Step 4: Add absolute ethanol to the system for dispersion, filter and collect the filter cake, add the filter cake to 150 ml of water and stir for 30 minutes to wash off the salt ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap