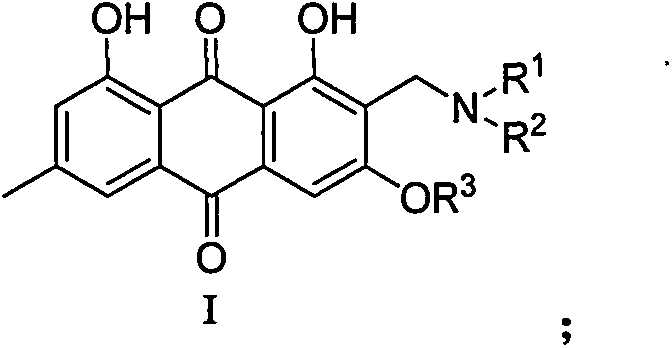
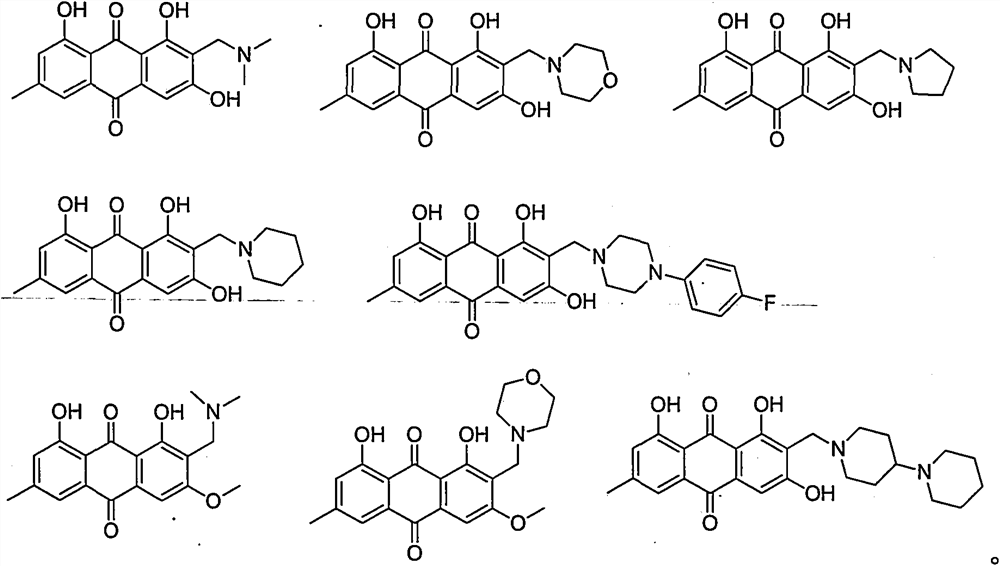
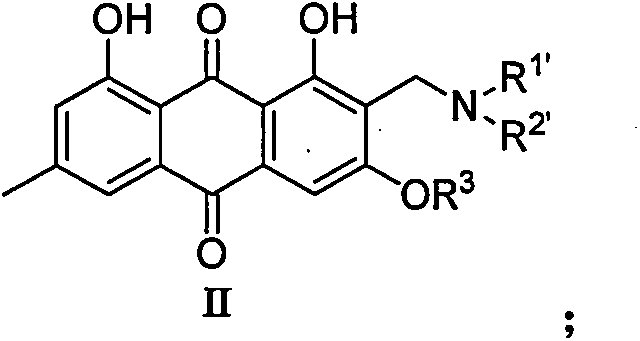
2-aminomethyl-9,10-anthraquinone derivative, its preparation method and application
An anthraquinone derivative, amine methyl technology, applied in the preparation of organic compounds, chemical instruments and methods, pharmaceutical formulations, etc., can solve the problems of general action strength, increase activity, etc., and achieve the effect of good anti-HCV activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Dissolve 5mmol of emodin methyl ether in 15mL of dioxane, add 10mmol of formaldehyde aqueous solution, 40mmol of acetic acid and 10mmol of dimethylamine, heat up to 85°C for 12 hours, evaporate the solvent under reduced pressure, add 15mL of saturated sodium bicarbonate aqueous solution, and then Extracted three times with 15 mL of dichloromethane, combined the organic phases, washed once with 15 mL of water, and recrystallized from ethyl acetate to obtain compound 1 with a yield of 75%. 2-(Dimethylamino)methyl-1,8-dihydroxy-3methoxy-6-methylanthraquinone-9,10-dione, 2-((dimethylamino)methyl)-1,8- dihydroxy-3-methoxy-6-methylanthracene-9, 10-dione;
[0059] 1 H NMR (500MHz, CDCl 3 )δ12.28(s, 1H), 7.61(s, 1H), 7.41(s, 1H), 7.08(s, 1H), 4.02(s, 3H), 3.63(s, 2H), 2.45(s, 3H ), 2.34(s, 6H); 13 C NMR (126MHz, CDCl 3 )δ190.8, 182.3, 164.5, 163.2, 162.5, 148.2, 134.4, 133.0, 124.6, 121.0, 119.7, 113.8, 110.9, 102.8, 56.5, 50.4, 45.4, 22.1; ESI-MS: m / z 342.2 [M+ H] + . ...
Embodiment 2
[0061] The reaction conditions and operations were the same as in Example 1, except that dimethylamine was replaced by morpholine to obtain compound 2 with a yield of 69%.
[0062] 1 H NMR (500MHz, CDCl 3 )δ12.74(s, 1H), 12.11(s, 1H), 7.63(s, 1H), 7.24(s, 1H), 7.08(s, 1H), 3.99(s, 2H), 3.83(s, 3H ), 3.81(brs, 4H), 2.69(brs, 4H), 2.47(s, 3H); 13 CNMR (126MHz, CDCl 3 )δ190.9, 182.3, 166.6, 162.5, 162.1, 148.7, 134.9, 133.8, 124.9, 121.8, 120.5, 114.5, 112.9, 110.8, 108.9, 66.6, 55.9, 54.1, 53.4, 22.2; ESI-MS: m / z .2[M+H] + .
Embodiment 3
[0064] Dissolve 5 mmol emodin in 15 mL dioxane, add 10 mmol aqueous formaldehyde, 40 mmol acetic acid and 10 mmol 4-piperidinylpiperidine, heat up to 85°C for 12 hours, evaporate the solvent under reduced pressure, add 15 mL saturated sodium bicarbonate The aqueous solution was extracted three times with 15 mL of dichloromethane, the organic phases were combined, washed once with 15 mL of water, and recrystallized from ethyl acetate to obtain compound 3 with a yield of 41%.
[0065] 1 H NMR (500MHz, CDCl 3 )δ12.71(s, 1H), 12.13(s, 1H), 7.61(s, 1H), 7.14(s, 1H), 7.03(s, 1H), 3.97(s, 2H), 3.57-3.89(m , 8H), 2.64-2.91(m, 8H), 2.45(s, 3H), 1.75-1.85(m, 2H); 13 C NMR (126MHz, CDCl 3 )δ190.7, 181.5, 167.6, 163.5, 162.7, 147.9, 135.1, 133.2, 124.1, 121.1, 120.2, 115.1, 113.3, 111.2, 107.9, 71.1, 66.6, 57.9, 55.9, 52.1, 53.2, 25 ;ESI-MS: m / z 451.2 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com