A diazaspiro derivative containing 3-trifluoromethyl-phenyl substituent and its preparation method and application
A diazaspiro ring and phenyl substitution technology, applied in organic chemistry, antiviral agents, etc., can solve problems such as high adverse reactions, price restrictions, and shortened treatment cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
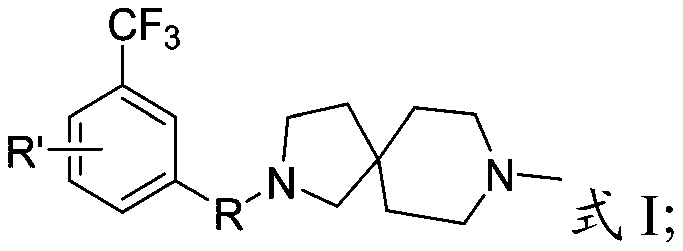
[0045] The present invention provides a preparation method for diazaspiro derivatives containing 3-trifluoromethyl-phenyl substituents described in the above technical scheme, comprising the following steps:
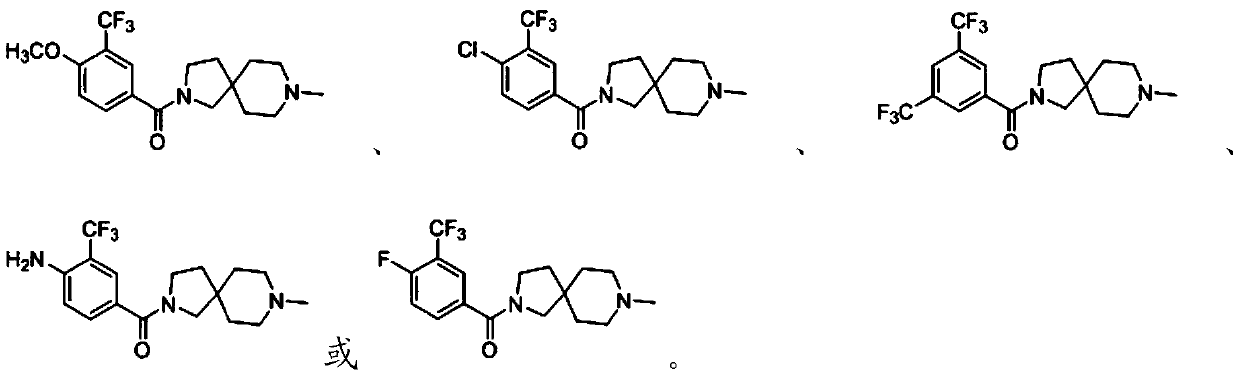
[0046] The compound containing 3-trifluoromethyl-phenyl substituent is subjected to substitution reaction with 8-methyl-2,8-diazaspiro[4.5]decane to obtain 3-trifluoromethyl-containing compound having the structure shown in formula I Diazaspiro derivatives of fluoromethyl-phenyl substituents;
[0047] The compound containing 3-trifluoromethyl-phenyl substituent has the structure shown in formula II:
[0048]
[0049] In formula II, R" is -Cl, -Br, -OH or
[0050] The present invention preferably selects the preparation method of the diazaspiro derivatives containing 3-trifluoromethyl-phenyl substituent according to R, specifically:
[0051] When R is -SO 2 -or-CH 2 -, in the presence of an organic solvent, compound 1 and 8-methyl-2,8-diazaspiro[4.5]decane are su...
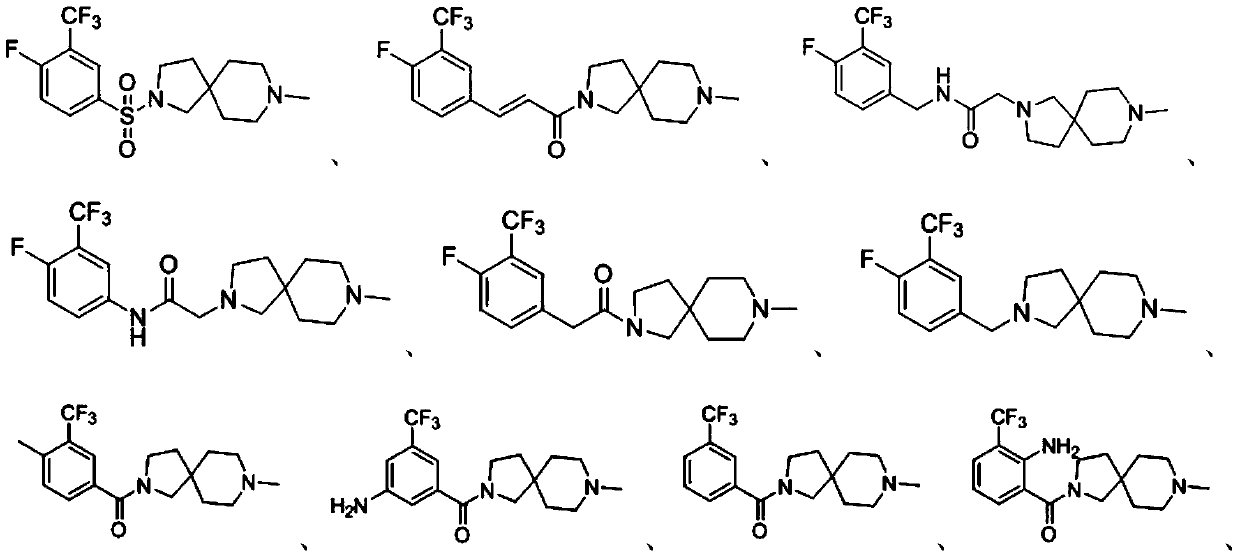
Embodiment 1
[0104] Preparation of compound A, the reaction process is as follows:
[0105]
[0106] At 0°C, add ultra-dry solvent dichloromethane (DCM, 5 mL), compound A-a (131 mg, 0.5 mmol) and 8-methyl-2,8-diazaspiro[ 4.5] Decane (154 mg, 1 mmol), reacted for 2.5 h (TLC tracking); the pH value of the resulting system was adjusted to 10 with 1 mol / L sodium hydroxide solution, and then DCM (15 mL) extraction, water (10 mL) Washing, saturated sodium bicarbonate solution (10mL), saturated brine (10mL), collecting the organic phase, using anhydrous sodium sulfate to dry the organic phase, filtering, and the obtained filtrate was concentrated under reduced pressure and then subjected to silica gel column chromatography separation and purification (The eluent was dichloromethane:methanol=20:1, v / v) to obtain Compound A (59.4 mg, yield 31.26%) as a milky white solid.
[0107] HRMS: Anal.calcd for C 16 h 21 f 4 N 2 o 2 S 381.12544. Found: 381.12620. 1 H-NMR (500MHz, DMSO-d 6 )δ8.24(s,...
Embodiment 2
[0109] Preparation of compound B, the reaction process is as follows:
[0110]
[0111] At 0°C, add ultra-dry solvent dichloromethane (DCM, 5mL), compound B-b (234mg, 1mmol) and N,N'-carbonyldiimidazole (194.4mg, 1.2mmol) into a dry one-necked bottle protected by nitrogen , reaction 2h (TLC tracking);
[0112] Add 8-methyl-2,8-diazaspiro[4.5]decane (184.8mg, 1.2mmol) to the resulting system, react at 25°C for 12h (TLC tracking); Sodium solution adjusted the pH value of the resulting system to 10, and then carried out DCM (15mL) extraction, water (10mL) washing, saturated sodium bicarbonate solution (10mL) washing, and saturated brine (10mL) washing in sequence, and collected the organic phase. The organic phase was dried with sodium sulfate water, filtered, and the resulting filtrate was concentrated under reduced pressure and purified by silica gel column chromatography (eluent: dichloromethane:methanol=5:1, v / v) to obtain milky white solid compound B ( 106.1mg, the yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com