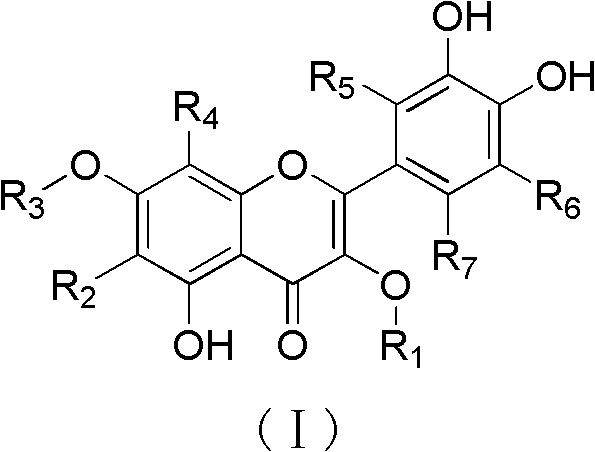
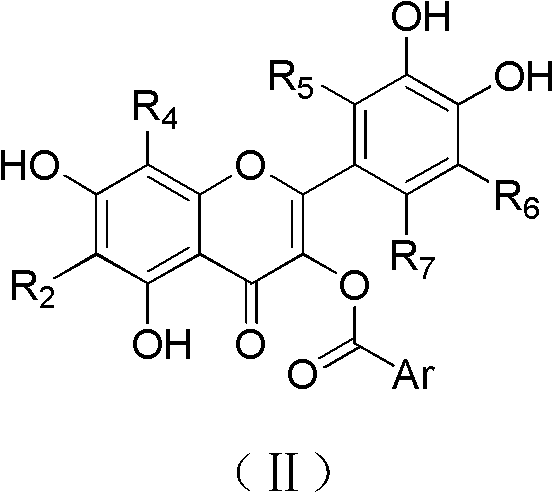
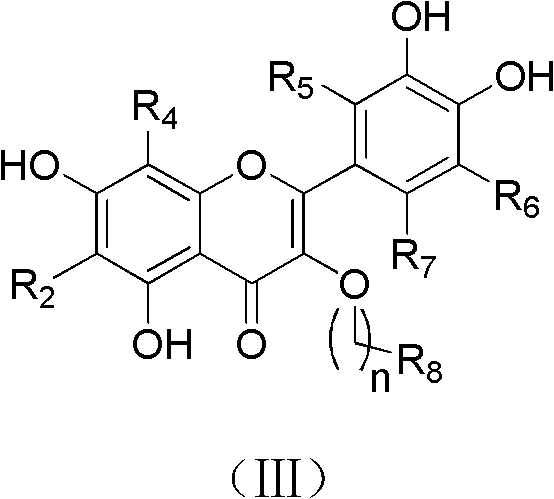
Quercetin derivatives or their analogs and applications thereof
A technology for quercetin and analogs, applied in the field of medicinal chemistry, can solve the problems of large toxic and side effects, expensive treatment costs, unsatisfactory treatment effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of 7-benzyloxy-2-(3,4-dibenzyloxyphenyl)-3,5-dihydroxy-4H-benzopyran-4-one.
[0029] Reaction 1
[0030]
[0031] Dissolve 5.00g of rutin (8.2mmol) and 2.83g of potassium carbonate (20.5mmol) in 40mL of anhydrous N,N-dimethylformamide (DMF), react for 0.5h under nitrogen protection, add 3.2mL of benzyl Bromine (27.1 mmol), continued to stir the reaction at 60°C for 3h. After the reaction was completed, the reaction solution was acidified with 10% acetic acid to pH=5, a solid precipitated out, and the precipitate was collected by centrifugation. 60 mL of ethanol was added to the above precipitate, and 9 mL of concentrated hydrochloric acid was added in portions, and the reaction solution was stirred and reacted at 70° C. for 2 h. After the reaction, cool to room temperature, filter and wash the precipitated precipitate with water to obtain a crude product. The above crude product was recrystallized in a mixed solvent of dichloromethane / ethanol t...
Embodiment 2
[0032] Example 2. Synthesis of quercetin-3-O-aromatic formate compounds.
[0033] Reaction 2:
[0034]
[0035] Table 1
[0036]
[0037]
Embodiment 21
[0038] Example 2.1 Synthesis of 2-(3,4-dihydroxyphenyl)-3-benzoyloxy-5,7-dihydroxy-4H-chromen-4-one (compound 1-1).
[0039] 200mg of the product 7-benzyloxy-2-(3,4-dibenzyloxyphenyl)-3,5-dihydroxy-4H-benzopyran-4-one (0.35mmol) obtained in Example 1 Mix with 100mg 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDCI, 0.525mmol), 9mg 4-dimethylaminopyridine (DMAP, 0.025mmol) and 43mg benzoic acid (0.35mmol), Stir in 20 mL of DMF at room temperature for 10 hours. After the reaction was completed, the reaction solution was poured into 100 mL of water and left to stand for 1 hour, a large amount of precipitates were precipitated, filtered and dried to obtain a light yellow solid crude product. The above crude product was dissolved in 20 mL of ethanol / dioxane (3 / 1) solution, 20 mg of 10% palladium-carbon was added, and the reaction was carried out in a hydrogen atmosphere for 3 hours. After the reaction was completed, filter, the filter cake was washed with ethanol, the filtrate w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com