Preparation method of borosilicate glass ceramic solidified body containing uranpyrochlore
A borosilicate glass and ceramic solidification technology, which is applied in the field of radioactive waste treatment, can solve the problems of limited containment capacity, limited proportion of ceramic phase, and the inability of the solidified body to handle high-level radioactive waste to the greatest extent, so as to increase the containment capacity, The effect of uniform distribution of ceramic phase and compact structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
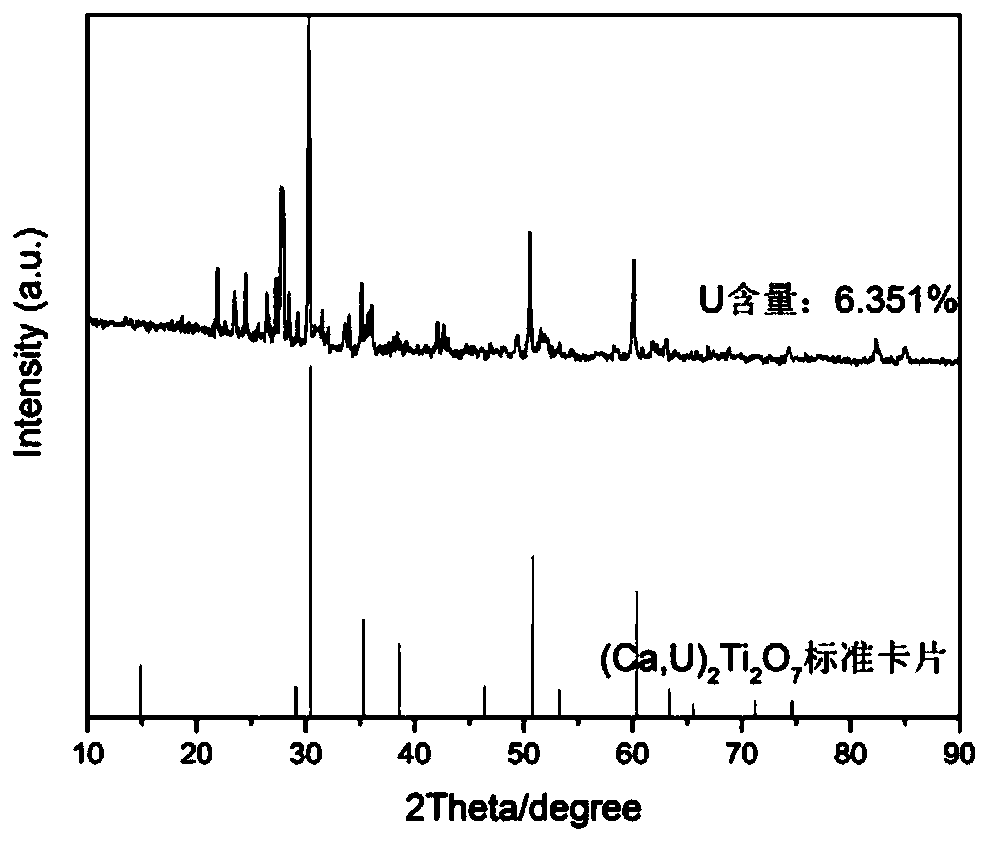
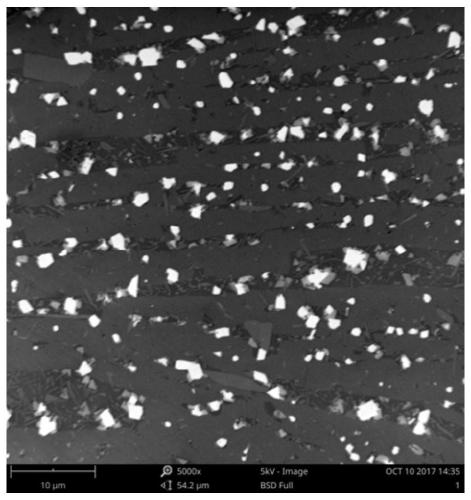
[0035] A method for preparing a borosilicate glass ceramic solidified body containing uranium pyrochlore in this embodiment, step 1, weighing the following components by weight percentage: SiO 2 20.5%, Al 2 o 3 4%, Na 2 CO 3 9%, B 2 o 3 4%, CaF 2 17.5%, CaO4%, U 3 o 8 25.5%, TiO 2 15%;
[0036] Step 2. Pretreatment of glass-ceramic raw materials
[0037] SiO 2 、Al2 o 3 、Na 2 CO 3 , B 2 o 3 , CaF 2 Wet-mix and dry the six components of CaO to obtain a uniformly mixed first mixture, and U 3 o 8 and TiO 2 The two components were wet-mixed and dried to obtain a uniformly mixed second mixture. The first mixture and the second mixture were respectively sintered at 650° C. for 1 h under an argon atmosphere, and then the sintered first mixture and The second mixture is ball milled for 3 hours respectively, the first mixture after ball milling is the first glass-ceramic raw material, and the second mixture after ball-milling is the second glass-ceramic raw material...
Embodiment 2
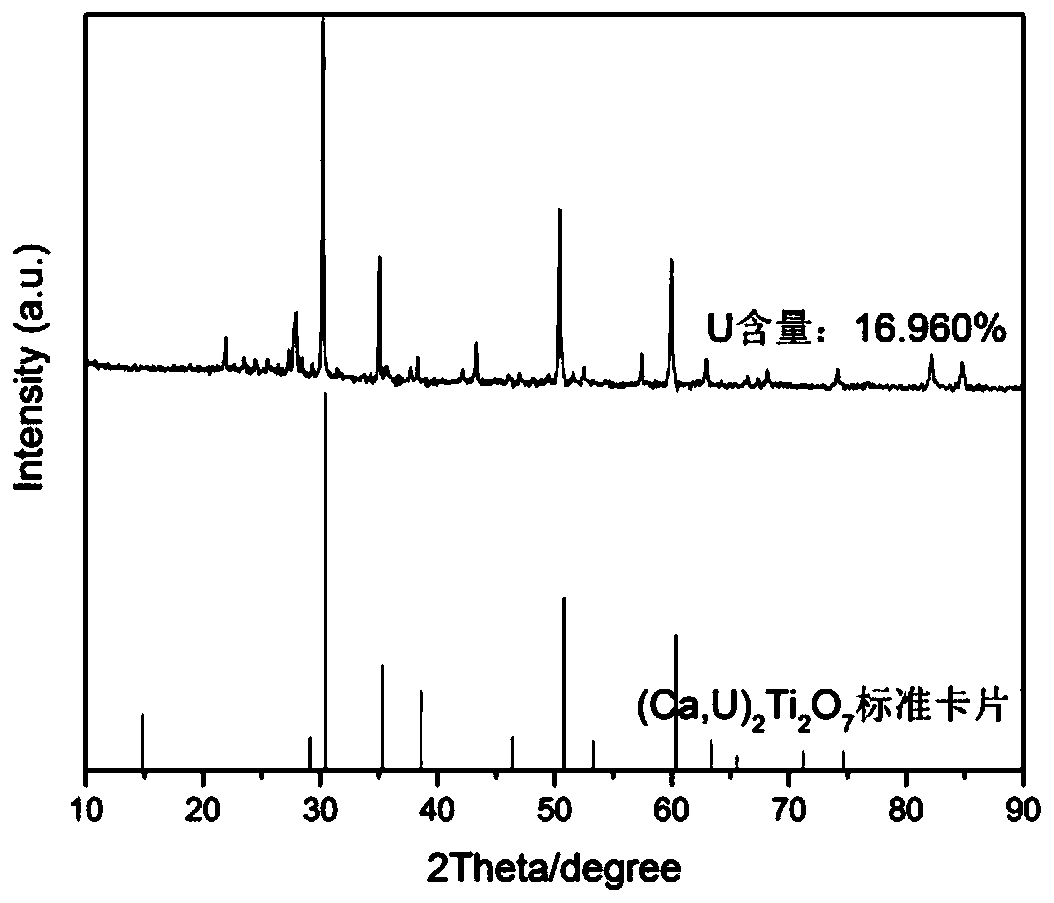
[0048] A method for preparing a borosilicate glass ceramic solidified body containing uranium pyrochlore in this embodiment, step 1, weighing the following components by weight percentage: SiO 2 20%, Al 2 o 3 2%, Na 2 CO 3 8%, B 2 o 3 3%, CaF 2 17%, CaO3%, U 3 o 8 30%, TiO 2 16%;
[0049] Step 2. Pretreatment of glass-ceramic raw materials
[0050] SiO 2 、Al 2 o 3 、Na 2 CO 3 , B 2 o 3 , CaF 2 Wet-mix and dry the six components of CaO to obtain a uniformly mixed first mixture, and U 3 o 8 and TiO 2 The two components were wet mixed and dried to obtain a uniformly mixed second mixture. The first mixture and the second mixture were respectively sintered at 750° C. for 3 hours under an argon atmosphere, and then the sintered first mixture and The second mixture is ball milled for 4 hours respectively, the first mixture after ball milling is the first glass-ceramic raw material, and the second mixture after ball-milling is the second glass-ceramic raw material...
Embodiment 3
[0066] A method for preparing a borosilicate glass ceramic solidified body containing uranium pyrochlore in this embodiment, step 1, weighing the following components by weight percentage: SiO 2 23.5%, Al 2 o 3 2.1%, Na 2 CO 3 8.1%, B 2 o 3 3.1%, CaF 2 20%, CaO3.1%, U 3 o 8 20.1%, TiO 2 20%;
[0067] Step 2. Pretreatment of glass-ceramic raw materials
[0068] SiO 2 、Al 2 o 3 、Na 2 CO 3 , B 2 o 3 , CaF 2 Wet-mix and dry the six components of CaO to obtain a uniformly mixed first mixture, and U 3 o 8 and TiO 2 The two components were wet-mixed and dried to obtain a uniformly mixed second mixture. The first mixture and the second mixture were respectively sintered at 700° C. for 2 hours under an argon atmosphere, and then the sintered first mixture and The second mixture is ball milled for 3.5 hours respectively, the first mixture after ball milling is the first glass-ceramic raw material, and the second mixture after ball-milling is the second glass-cerami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com