Bcl-2 protein inhibitor and preparation method and application thereof
A technology represented by C3-C8, applied in the field of medicine, can solve the problems of failing to meet the needs of clinical application and prone to drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] Two, the preparation method of Bcl-2 protein inhibitor
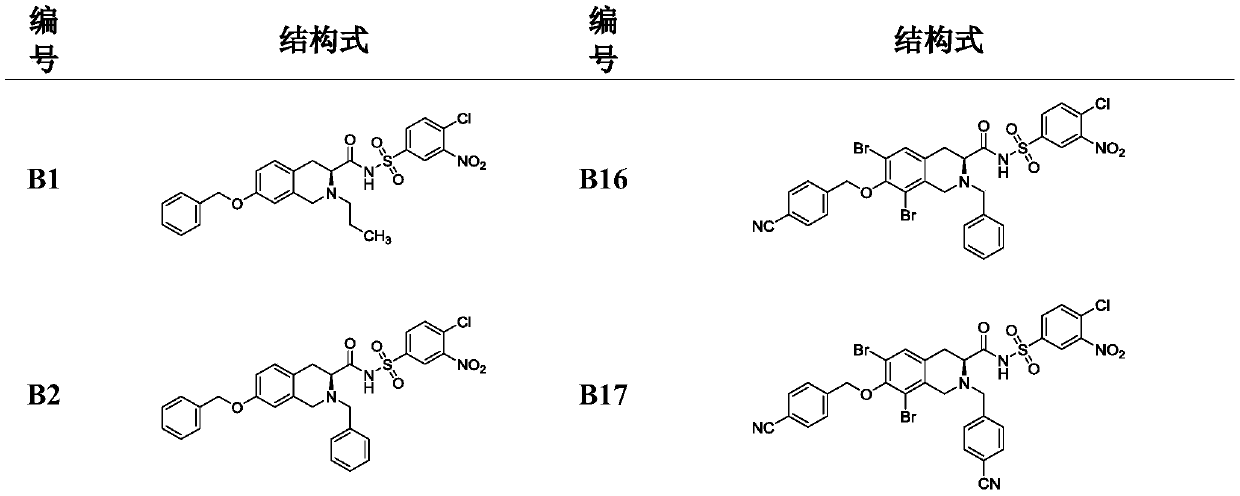
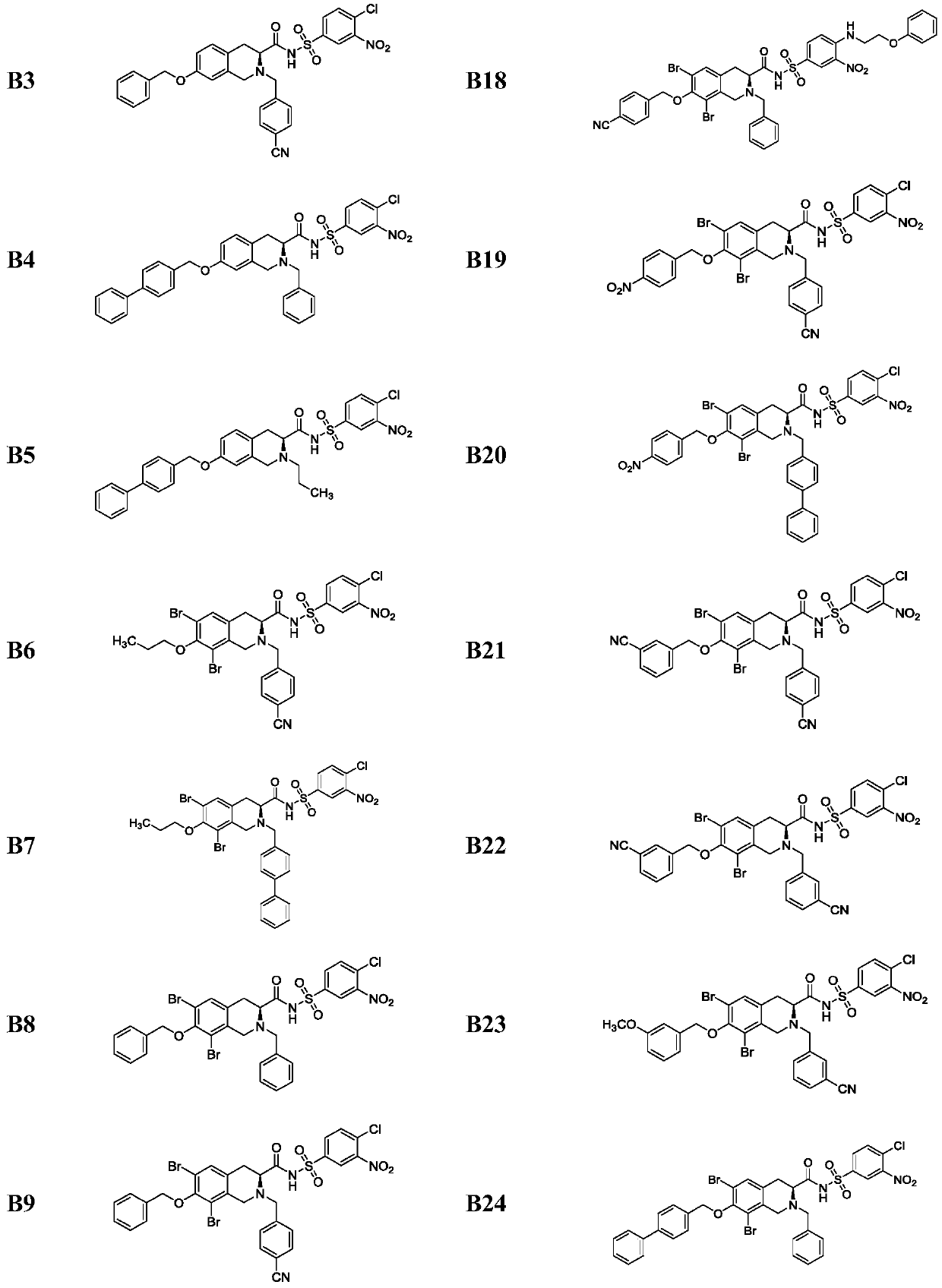
[0058] A method for preparing a Bcl-2 protein inhibitor, the steps comprising: the starting material L-tyrosine 1 undergoes dibromination to obtain an intermediate 2, and the intermediate 2 obtains a key intermediate 3 through a Pictet-Spengler reaction; the intermediate 3 Methylation, N-Boc protection gives intermediate 4; intermediate 4 hydrodebromination, O-alkylation and de-Boc protection give intermediates 5a and 5b, or only O-alkylation and de-Boc protection Intermediates 5c-5k are obtained; intermediates 5a-5k are N-alkylated to obtain 6a-6w; intermediates 6a-6w are hydrolyzed with methyl esters, and reacted with different benzenesulfonamides by mixed anhydride method to obtain compounds shown in B1-B30 .
[0059] The synthetic route is as follows:
[0060]
[0061] Among them, R 1 , R 2 , R 3 is as defined above;
[0062] Reagents and conditions: a) bromine, glacial acetic acid, room temperature; ...
Embodiment 1
[0102] Example 1. (S)-7-(benzyloxy)-N-((4-chloro-3-nitrophenyl)sulfonyl)-2-propyl-1,2,3,4-tetrahydro Synthesis of Isoquinoline-3-carboxamide (B1)
[0103] Synthesis of (S)-2-amino-3-(3,5-diiodo-4-hydroxyphenyl)propionic acid hydrobromide (2)
[0104] Dissolve 10.00 g of L-tyrosine in 100 mL of glacial acetic acid, slowly add 20 mL of glacial acetic acid solution containing 26.46 g of bromine dropwise, and react at room temperature for 4 hours; spin off the glacial acetic acid, and wash the crude product with ether to obtain 21.21 g of a white solid. Yield 96%. 1 H NMR (400MHz, DMSO-d 6 )δ13.94(s,1H),9.90(s,1H),8.25(s,3H),7.45(s,2H),4.23(t,J=6.0Hz,1H),3.06(dd,J=14.5 ,5.8Hz,1H),2.99(dd,J=14.5,6.9Hz,1H).
[0105] Synthesis of (S)-6,8-diiodo-7-hydroxyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylate hydrochloride (3)
[0106] Dissolve 21.21g of compound 2 in 190mL of concentrated hydrochloric acid, add 15.5mL of ethylene glycol dimethyl ether and 5.90g of paraformaldehyde in seq...
Embodiment 2
[0118]Example 2. (S)-2-Benzyl-7-(benzyloxy)-N-((4-chloro-3-nitrophenyl)sulfonyl)-1,2,3,4-tetrahydro Synthesis of Isoquinoline-3-carboxamide (B2)
[0119] The preparation methods of intermediates and target compounds are as in Example 1. Yield 54%, mp: 176-178°C. 1 H NMR (400MHz, DMSO-d 6 )δ9.99(s,1H),8.34(d,J=1.7Hz,1H),8.04–7.88(m,1H),7.83(d,J=8.4Hz,1H),7.52–7.24(m,10H ),7.17(d,J=8.1Hz,1H),6.99–6.80(m,2H),5.07(s,2H),4.34(d,J=12.7Hz,1H),4.20(s,2H),4.18 –4.07(m,1H),4.07–3.90(m,1H),3.23(dd,J=17.0,5.5Hz,1H),3.11(dd,J=17.0,8.8Hz,1H).HRMS(AP-ESI )m / z Calcd for C 30 h 26 ClN 3 o 6 S[M-H] - 590.1153,found:590.1160.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap