Structure, preparation method and application of quinoxalinone derivative
A kind of technology of quinoxalinone and derivatives, applied in the fields of structure, preparation and use of quinoxalinone derivatives, can solve problems such as insufficient active strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
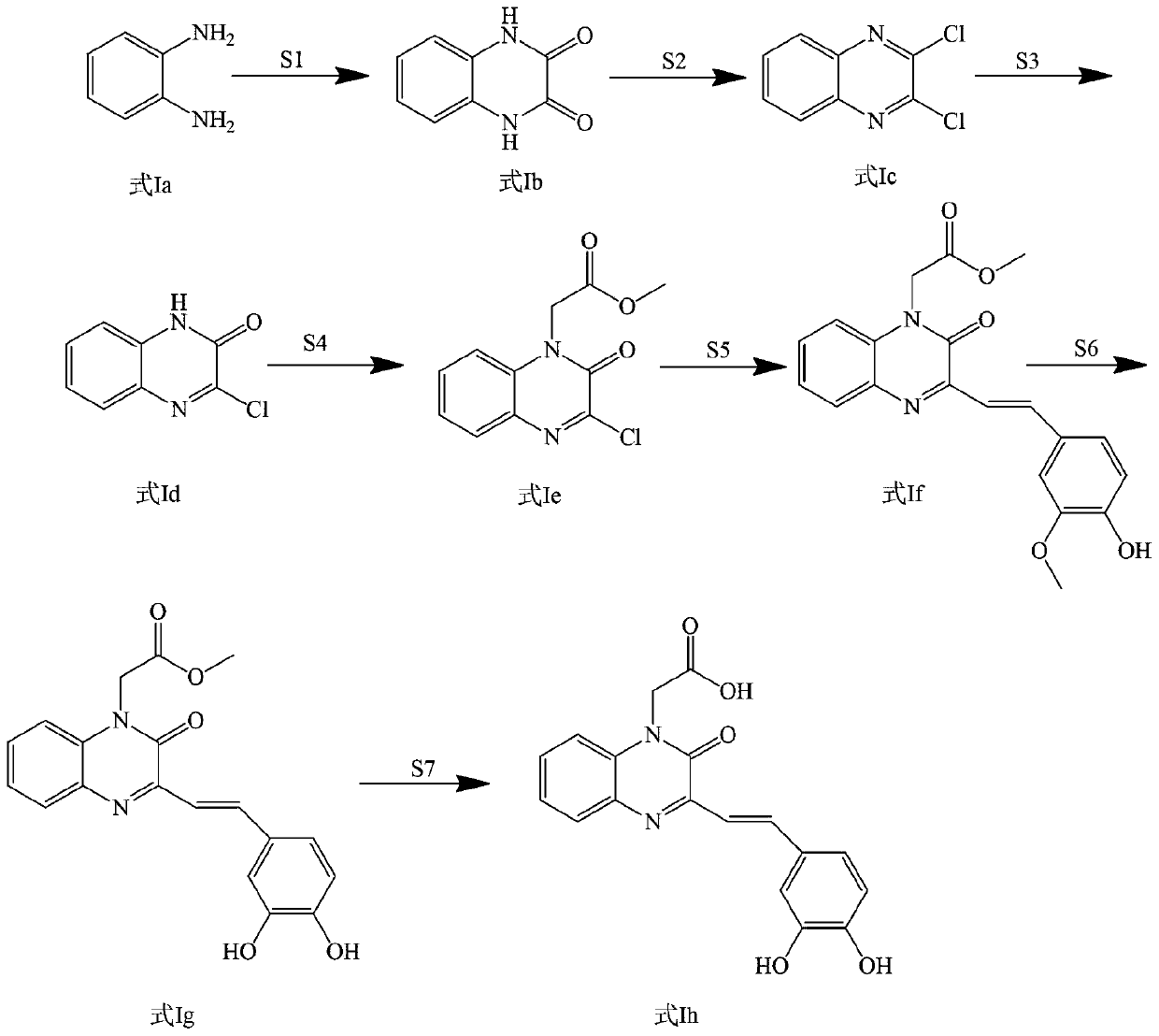
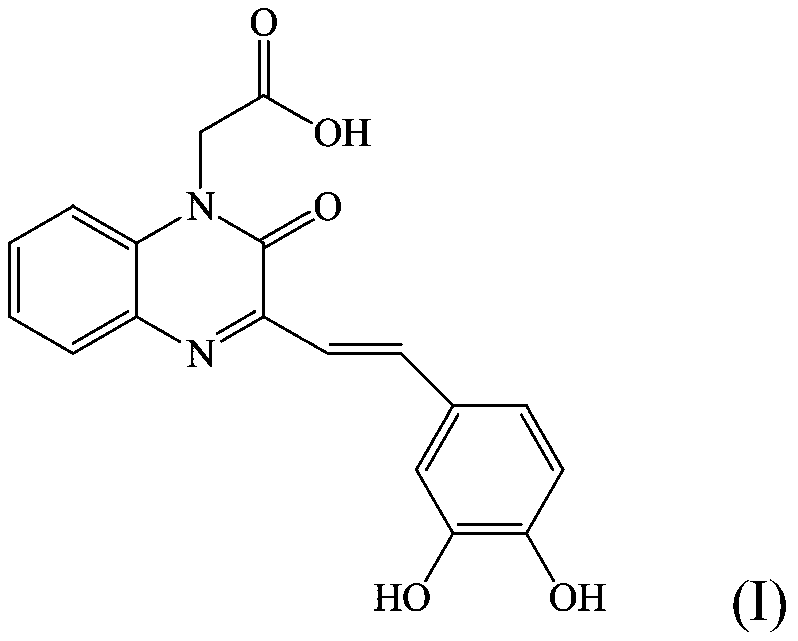
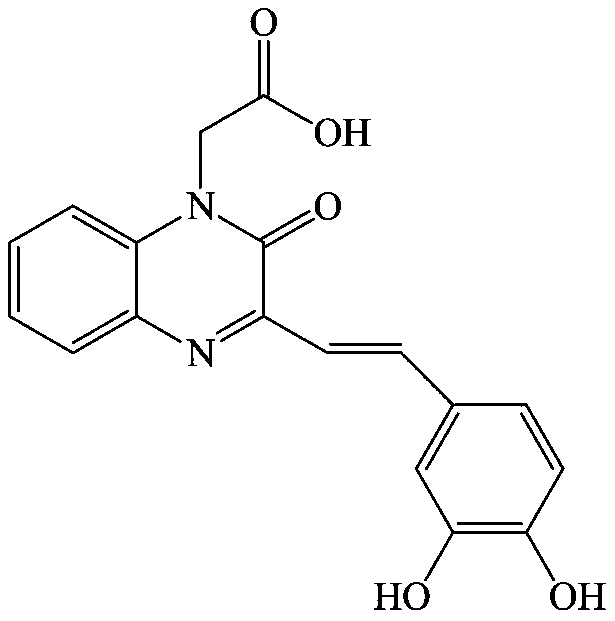
[0047] Example 1: Preparation of 2-(3-(3,4-dihydroxystyryl)-2oxaquinoxaline-1(2H)-alkyl)acetic acid synthesis (compound 1)
[0048]
[0049] Add 4.475g o-phenylenediamine and 6.752g oxalic acid into a 250mL flask and mix, add 50mL water to dissolve, then add 5mL concentrated hydrochloric acid to acidify, heat to 100℃, stir for 6h, use thin layer chromatography (TLC monitoring) After judging that the raw material o-phenylenediamine disappeared completely, the reaction was stopped. After the reaction solution is cooled to room temperature, vacuum pump is used to filter, and the filtrate is washed with water until the pH is neutral. 4 After drying, it was filtered again to obtain the compound quinoxaline-2,3(1H,4H)-dione (white crystals, yield 7.476g, yield 92%) 1 H NMR(400MHz,[D 6 ]DMSO): δ7.065 (d, 2H, J=6.4 Hz), 7.115 (d, 2H, J=6.4 Hz), 11.894 (s, 2H).
[0050] Weigh 4.054g quinoxaline-2,3(1H,4H)-dione, measure 3.63ml SOCl 2 Mix the two and add them to a 250mL flask, and then measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com