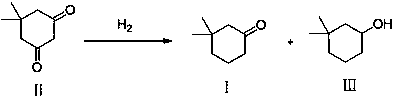
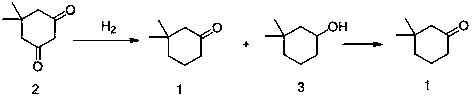
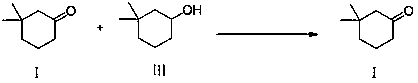
Synthetic method of 3,3-dimethylcyclohexanone
A technology of dimethylcyclohexanone and a synthesis method, applied in the field of drug synthesis, can solve the problems of serious corrosion of equipment, difficult purification and the like, and achieve the effects of low cost, easy scale-up production and alleviation of purification difficulty.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of Compounds 1 and 3
[0028] Add 10.0 g of compound 2 to 100 mL of isopropanol and 0.5 g of 5% Pd / C. React at 65°C for 48 hours under 2 MPa hydrogen pressure. The Pd / C was removed by filtration, and the reaction solution was concentrated to obtain a mixture of compound 1 and compound 3 (the ratio on GC was about 17:1), and the subsequent reaction was carried out directly without separation.
Embodiment 2
[0029] Example 2 Preparation of compound 1
[0030] Add 100 mL of dichloromethane and 100 mL of water to the mixture obtained in Example 1 above. Add 14.7 g sodium bromide, 9 g sodium bicarbonate. Stir and cool down to 0~10°C, add 0.2 g TEMPO, and dropwise add 101 g 5% sodium hypochlorite aqueous solution to react. Complete conversion of compound 3 to compound 1 was detected by GC. The crude product of compound 1 was obtained after separation and washing with water, and the GC purity was as high as 95%. Compound 1 (3,3-dimethylcyclohexanone) was obtained after distillation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com