Liquid oral pharmaceutical dosage form comprising a histamine h2-receptor antagonist and an antacid
A receptor antagonist, pharmaceutical dosage form technology, applied in the field of liquid oral pharmaceutical dosage forms, can solve the problems of unstable famotidine, no suspension guidance and examples are given, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Material
[0054] All oils were obtained from Croda International.
[0055] Magnesium hydroxide and calcium carbonate were obtained from Magnesia GmbH.
[0056] Famotidine was obtained from Gedeon Richter, Hungary, and was internally film coated using conventional coating techniques well known to those skilled in the art.
[0057] preparation
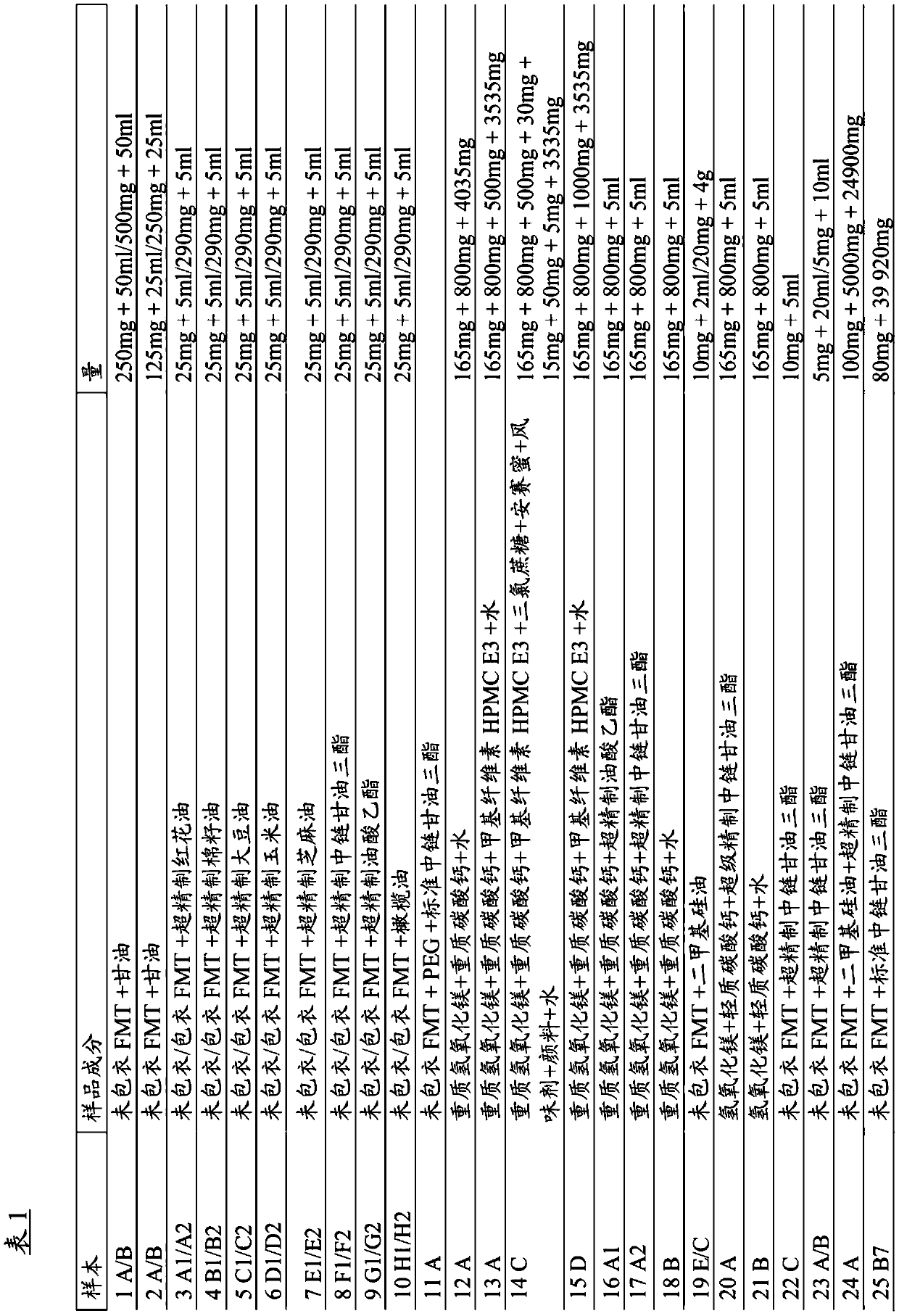
[0058] Different kinds of formulations were prepared and shown in Table 1.
[0059] Famotidine was mixed in different kinds of solvents, including different oils, to investigate which oils were suitable for use with famotidine (Samples 1-11 and 22-25 in Table 1). The results show that famotidine is stable in all oils and is most stable in the presence of medium chain triglycerides (samples 8F1 / F2, 22C, 23A / B, 24A and 25B7).
[0060] Different kinds of antacid mixtures were investigated to determine which might be suitable (samples 12-21 in Table 1).
[0061] Two-compartment packaging
[0062] A two-compartment pack is...
Embodiment 2
[0065] Analysis of the stability of famotidine suspended in different oils.
[0066] Mix uncoated famotidine in one of the following oils: safflower oil (B1), soybean oil (B2), sesame oil (B3), corn oil (B4), cottonseed oil (B5), ultra Refined MCT (B6) and Standard MCT (B7). 3 samples were prepared per batch and the samples were incubated at 40 / 75°C, 50°C or 60°C. 10 mg of famotidine was mixed with 5 g of oil. Samples were taken after 14 days, 1 month, 2 months, and 3 months and famotidine stability was assessed.
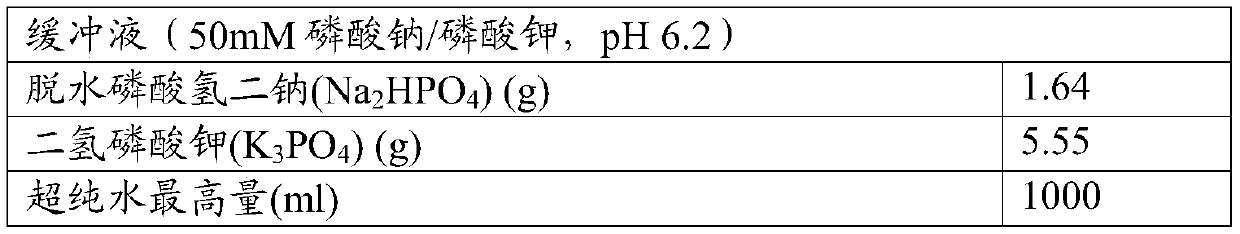
[0067] For stability analysis, samples were prepared and analyzed using the following methods
[0068] solution
[0069]
[0070]
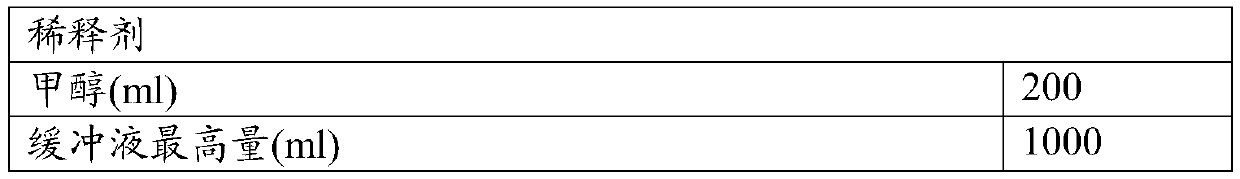
[0071] mobile phase
[0072]
[0073]
[0074] Famotidine Standard
[0075]
[0076] Famotidine: CAS Number: 76824-35-6, C 8 H 15 N 7 O 2 S 3 , MW: 337.45
[0077]
[0078]
[0079]
[0080]
[0081]
[0082] Sample Preparation
[0083] • Pour the sample vial into a 100ml volumetr...
Embodiment 3
[0102] Evaluate the viscosity and consistency of different antacid formulations.
[0103] Different amounts of Xanthan Gum 180 and Avicel CL 611 NF were used.
[0104]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com