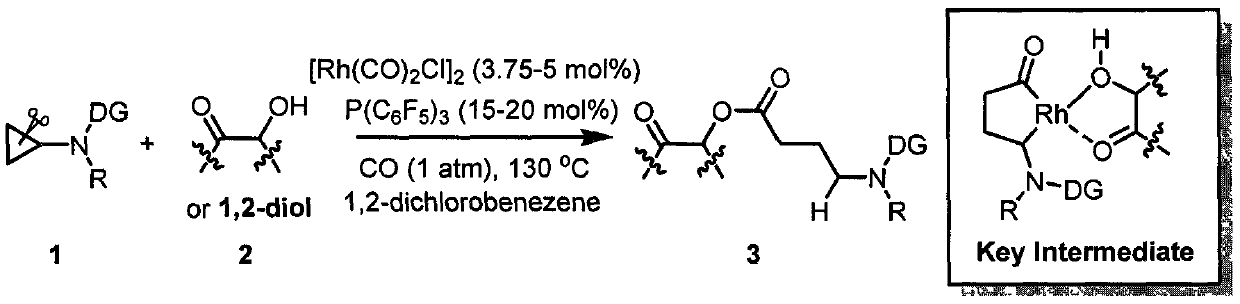
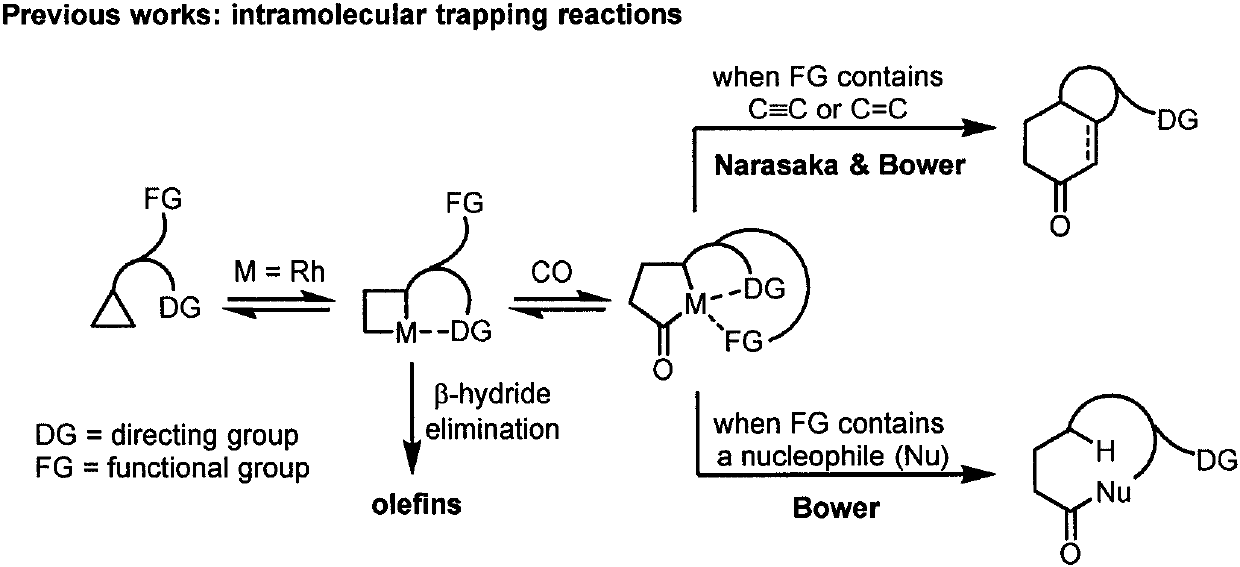
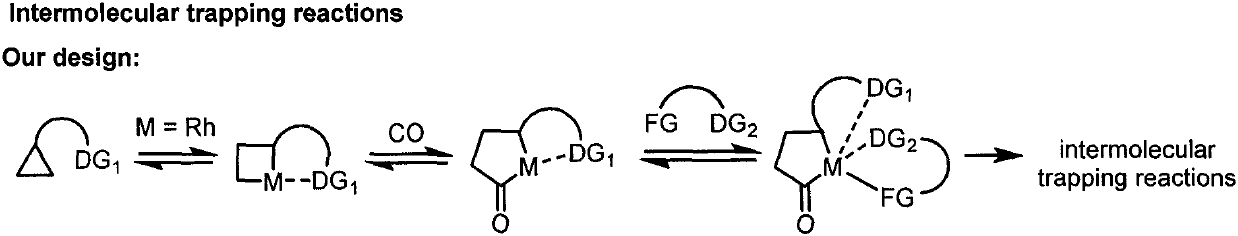
Efficiently synthesized gamma-aminobutyrate derivative with weak activated cyclopropane and alcohol intermolecular carbonylation coupling
A technology of carbonylation coupling and aminobutyrate, applied in the field of application, can solve the problems of poor substrate diversity and applicability, cumbersome synthesis steps, etc., and achieve the effects of good reaction applicability, wide range of substrates, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Synthesis of 2-Oxo-2-phenylethyl 4-(1,3,3-Trimethylureido)butanoate
[0025]
[0026] Add raw materials 1a (142mg, 1.0mmol), 2a (272mg, 2.0mmol), [Rh(CO) 2 Cl] 2 (14.5mg, 0.0375mmol), P(C 6 f 5 ) 3 (80mg, 0.15mmol), 1,2-dichlorobenzene (5mL), 130°C, carbon monoxide for 36 hours. After the reaction was completed, silica gel column chromatography was carried out with eluent (ethyl acetate / petroleum ether=1 / 1) to obtain 244 mg of product 3a (colorless oily liquid), with a yield of 80%. 1 H NMR (400MHz, CDCl 3 )δ7.91(d, J=7.2Hz, 2H), 7.61(t, J=7.4Hz, 1H), 7.49(t, J=7.4Hz, 2H), 5.35(s, 2H), 3.25(t, J=7.3Hz, 2H), 2.83(s, 3H), 2.80(s, 6H), 2.50(t, J=7.4Hz, 2H), 2.0-1.93(m, 2H). 13 C NMR (101MHz, CDCl 3 )δ191.97, 172.44, 165.28, 133.93, 133.74, 128.70, 127.55, 65.85, 49.23, 38.48, 36.56, 30.91, 22.59. HRMS (ESI) calcd.for C 16 h 23 N 2 o 4 (M+H) + : 307.1652, Found: 307.1651.
Embodiment 2
[0027] Embodiment 2: the synthesis of Formestane Product
[0028]
[0029] Add raw material 1a (14.2mg, 0.1mmol), 2b (60mg, 0.2mmol), [Rh(CO) 2 Cl] 2 (1.45mg, 0.00375mmol), P(C 6 f 5 ) 3 (8mg, 0.015mmol), 1,2-dichlorobenzene (0.5mL), 130°C, carbon monoxide for 96 hours. After the reaction was completed, silica gel column chromatography was performed with eluent (ethyl acetate / petroleum ether=2 / 1) to obtain 26 mg of product 3b (colorless oily liquid), with a yield of 55%. 1 H NMR (400MHz, CDCl 3 )δ3.23(t, J=7.3Hz, 2H), 2.80(s, 3H), 2.77(s, 6H), 2.71-2.67(m, 1H), 2.51-2.41(m, 5H), 2.12-1.99 (m, 3H), 1.96-1.91(m, 4H), 1.86-1.67(m, 4H), 1.58-1.52(m, 1H), 1.44-1.38(m, 1H), 1.30-1.23(m, 5H) , 1.09-1.02(m, 2H), 0.89(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ220.29,190.45,171.10,165.54,155.17,139.29,53.87,50.81,49.44,47.53,39.23,38.75,36.88,35.81,34.74,34.66,33.41,31.32,30.96,29.83,23.94,22.84,21.79,20.34 , 17.76, 13.78.HRMS(ESI)calcd.forC 27 h 41 N 2 o 5 (M+H) + : 473.3010...
Embodiment 3
[0030] Embodiment 3: Synthesis of 16a-Hydroxyestrone Product
[0031]
[0032] Add raw material 1a (14.2mg, 0.1mmol), 2c (57mg, 0.2mmol), [Rh(CO) 2 Cl] 2 (1.9mg, 0.005mmol), P(C 6 f 5 ) 3 (10.6mg, 0.02mmol), 1,2-dichlorobenzene (0.5mL), 130°C and carbon monoxide for 96 hours. After the reaction was completed, silica gel column chromatography was performed with eluent (ethyl acetate / petroleum ether=2 / 1) to obtain 30 mg of product 3c (colorless oily liquid), with a yield of 65%. 1 H NMR (400MHz, CDCl 3)δ7.12(d, J=8.4Hz, 1H), 6.66(dd, J=8.4, 2.6Hz, 1H), 6.60(d, J=2.6Hz, 1H), 6.31-6.27(br, 1H), 5.47(d, J=8.3Hz, 1H), 3.23(td, J=7.1, 3.1Hz, 2H), 2.86-2.78(m, 11H), 2.41-2.36(m, 3H), 2.27-2.20(m, 1H), 2.17-2.10(m, 1H), 1.95-1.87(m, 4H), 1.82-1.74(m, 2H), 1.60-1.54(m, 2H), 1.52-1.37(m, 2H), 0.99( s, 3H). 13 C NMR (101MHz, CDCl 3 )δ213.98,172.55,165.64,154.88,137.46,130.48,126.21,115.44,113.13,72.47,49.44,47.95,47.65,43.75,38.75,38.26,36.81,31.30,31.27,29.39,29.19,26.30,25....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com