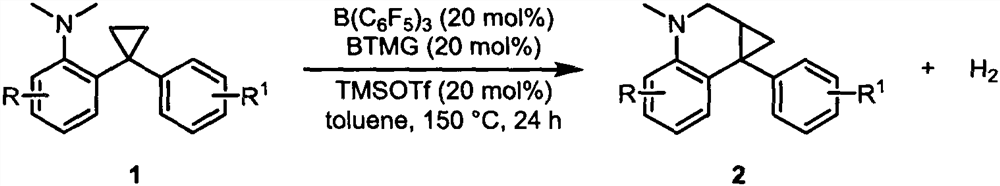
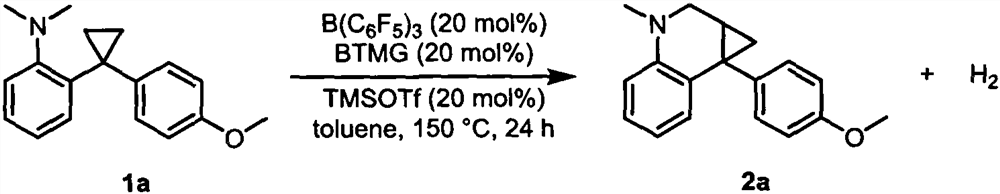Method for synthesizing tetrahydroquinoline compounds by taking inert cyclopropane as raw material
A technology of tetrahydroquinoline and cyclopropane, applied in the application field, can solve the problems of limited reaction type of inert cyclopropane, poor functional group tolerance, single reaction type, etc., and achieves good catalytic effect, simple operation and wide range of substrates. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of 7b-(4-methoxyphenyl)-3-methyl-1a, 2,3,7b-tetrahydro-1H-cyclopropa[c]Quinoline
[0026]
[0027] Under nitrogen atmosphere, in the reaction bottle of 15mL, raw material 1a (26.7mg, 0.1mmol), B(C 6 f 5 ) 3 (10.24mg, 0.02mmol), dissolved in dry toluene (1mL), then added BTMG (3.42mg, 0.02mmol), TMSOTf (4.44mg, 0.02mmol) and reacted at 150°C for 24 hours. After the reaction was completed, it was separated by silica gel column chromatography, and the eluent was (petroleum ether / ethyl acetate=100 / 1) to obtain 17.2 mg of product 2a (white solid), with a yield of 65%. 1 H NMR (400MHz, CDCl 3 ( s, 3H), 3.35(d, J=10.4Hz, 1H), 3.25(d, J=10.4Hz, 1H), 2.85(s, 3H), 1.81-1.80(m, 1H), 1.72-1.69(m , 1H), 1.32-1.29(m, 1H); 13 C NMR (101MHz, CDCl 3 )δ158.4, 144.5, 136.9, 131.9, 130.3, 128.5, 126.1, 117.7, 113.8, 111.3, 77.48, 55.4, 48.5, 39.2, 27.9, 27.1, 13.8. HRMS (ESI) m / z calcd for C 18 h 20 NO + (M+H) + 266.1539, found 266.1542.
Embodiment 2
[0028] Example 2: Synthesis of 2-(1-(4-methoxyphenyl)cyclopropyl)-N,N,5-trimethylaniline
[0029]
[0030] Under nitrogen atmosphere, in the reaction bottle of 15mL, raw material 1b (28.1mg, 0.1mmol), B(C 6 f 5 ) 3 (10.24mg, 0.02mmol), dissolved in dry toluene (1mL), then added BTMG (3.42mg, 0.02mmol), TMSOTf (4.44mg, 0.02mmol) and reacted at 150°C for 24 hours. After the reaction was completed, the product was separated by silica gel column chromatography, and the eluent was (petroleum ether / ethyl acetate=100 / 1) to obtain 15.6 mg of product 2b (white solid), with a yield of 56%. 1 H NMR (400MHz, CDCl 3 )δ7.29(d, J=8.2Hz, 1H), 6.98(d, J=8.6Hz, 2H), 6.82-6.81(m, 2H), 6.74(d, J=8.6Hz, 2H), 3.75( s, 3H), 2.60 (s, 6H), 2.33 (s, 3H), 1.41-1.38 (m, 2H), 1.28-1.25 (m, 2H); 13 C NMR (101MHz, CDCl 3 )δ157.1, 153.4, 139.8, 137.2, 134.3, 133.4, 126.8, 122.7, 120.1, 113.4, 55.4, 44.4, 26.36, 21.5, 19.3. HRMS (ESI) m / z calcd for C 19 h 24 NO + (M+H) + 282.1852, found 282.1859....
Embodiment 3
[0031] Example 3: Synthesis of 7b-(4-(benzyloxy)phenyl)-3-methyl-1a, 2,3,7b-tetrahydro-1H-cyclopropa[c]Quinolone
[0032]
[0033] Under a nitrogen atmosphere, in a 15mL reaction flask, the raw material 1c (34.3mg, 0.1mmol), B(C 6 f5 ) 3 (10.24mg, 0.02mmol), dissolved in dry toluene (1mL), then added BTMG (3.42mg, 0.02mmol), TMSOTf (4.44mg, 0.02mmol) and reacted at 150°C for 24 hours. After the reaction was completed, it was separated by silica gel column chromatography, and the eluent was (petroleum ether / ethyl acetate=100 / 1) to obtain 21 mg of product 2c (white solid), with a yield of 62%. 1 H NMR (400MHz, CDCl 3 )δ7.51-7.49 (m, 2H), 7.43-7.40 (m, 2H), 7.40-7.36 (m, 3H), 7.12-7.08 (m, 1H), 7.02-7.0 (m, 2H), 6.74- 6.72(m, 1H), 6.68-6.62(m, 3H), 5.12(s, 2H), 3.39(d, J=11.5Hz, 1H), 3.29(d, J=11.5Hz, 1H), 2.89(s , 3H), 1.88-1.84(m, 1H), 1.77-1.75(m, 1H), 1.37-1.34(m, 1H); 13 C NMR (101MHz, CDCl 3 ) 157.6, 144.5, 137.2, 131.9, 130.2, 128.8, 128.5, 128.1, 127.7, 126.1, 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com