Metal affinity fusion protein tag and its application
A fusion protein and affinity technology, applied in the field of bioengineering, can solve the problems of affecting protein activity, affecting protein refolding stability, solubility, activity reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
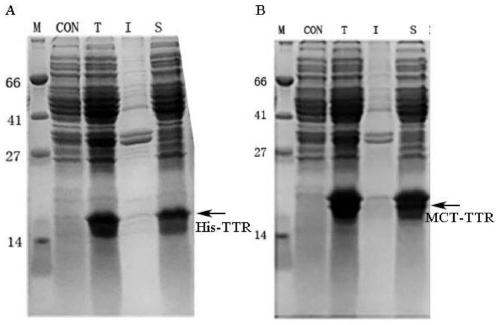
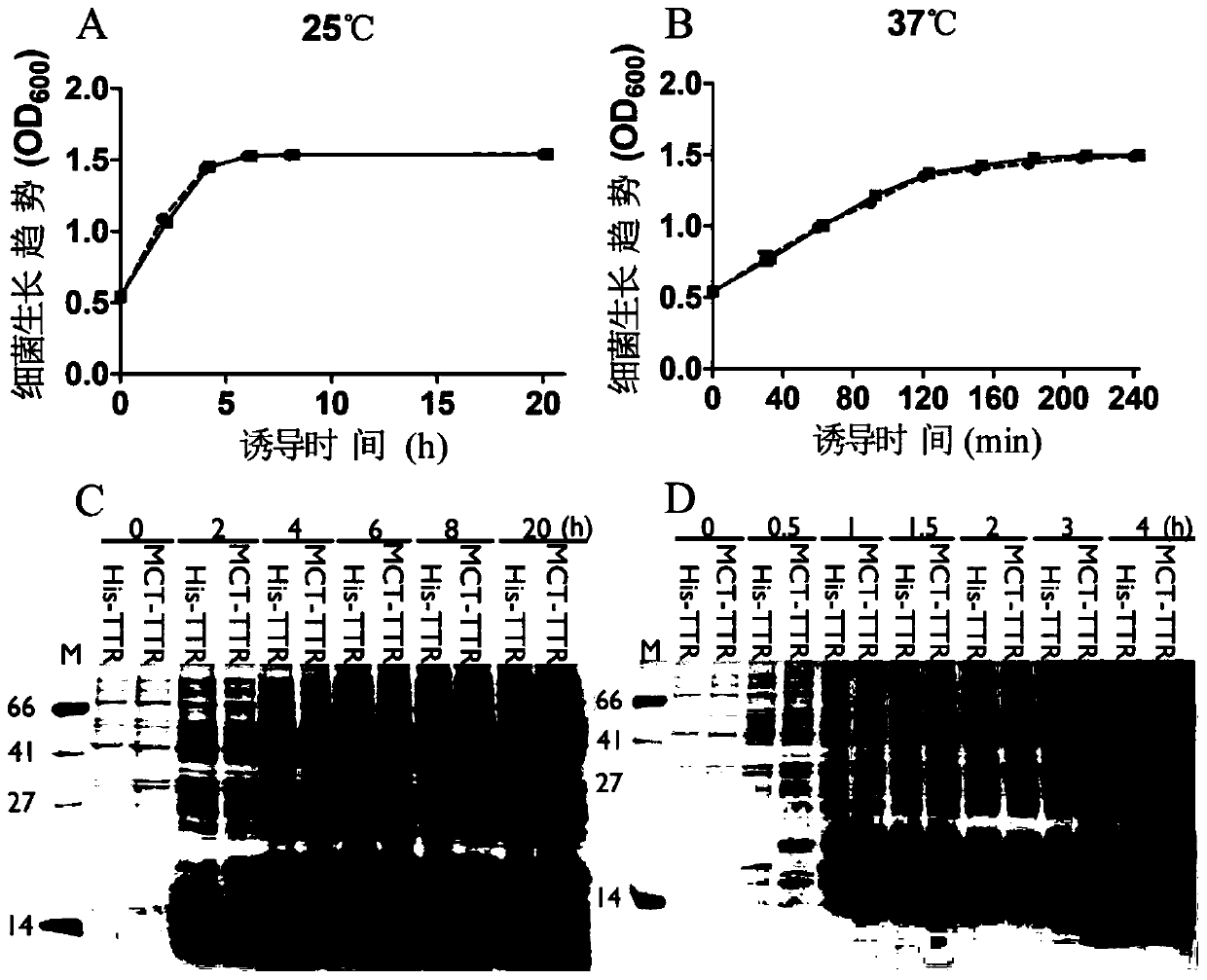
[0028] This patent provides a new short peptide sequence to replace His-tag. The sequence is selected from the amino-terminal 1-25 amino acid residues of the natural human copper ion transporter (MDHSHHMGMSYMDSNSTMQPSHHHP, SEQ ID No: 2), referred to as MCT-tag, which contains the ATCUN binding site and a methionine-rich region , and a stretch of three consecutive histidine residues. MCT-tag can be chelated with metal ions such as nickel and copper ions, so as to replace His-tag and fuse with the target protein, which is convenient for the purification of recombinant protein. MCT-tag is derived from human natural protein sequence, which can be retained on the target protein without removing MCT-tag, avoiding the complicated process of subsequent enzyme digestion and purification, improving production efficiency and reducing production costs. Unexpectedly, after MCT-tag fusion, the expression of the target protein is usually promoted, and the effect on the folding of the target...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com