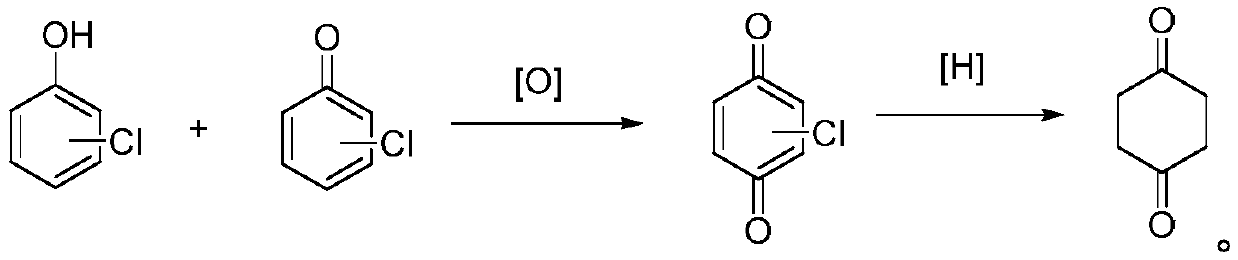
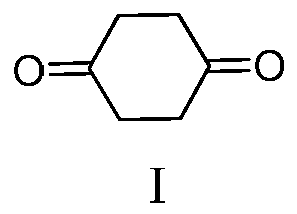
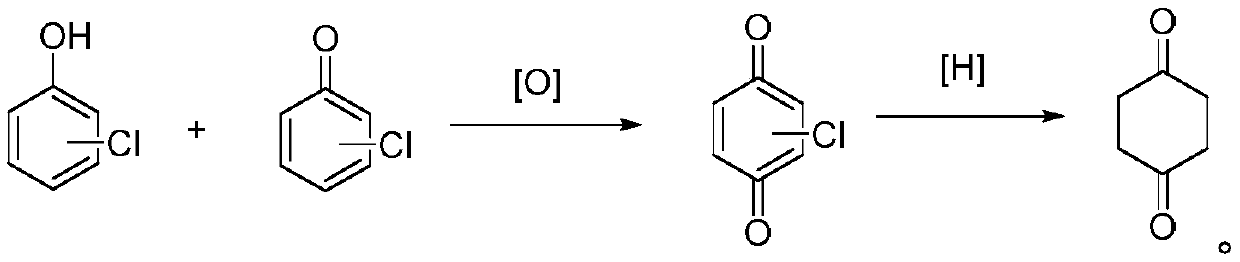
Preparation method of 1,4-cyclohexanedione
A technology of cyclohexanedione and dichlorophenol, which is applied in the field of preparation of chemical drug intermediates, can solve the problems of low product yield, high raw material price, and long reaction time, and achieve high product yield, low cost, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of chlorinated p-benzoquinone.
[0031] In a 1000mL sealed four-necked reaction flask, add 600g of methanol and 197.5g of 2,4,6-trichlorophenol, stir to dissolve, then in a 2000mL sealed four-necked reaction flask, add 200mL of 5% dilute nitric acid, start The dissolved 2,4,6-trichlorophenol solution was added dropwise, 2 atm of oxygen was introduced, the temperature was stirred at 20°C, and the reaction was carried out for 2 hours. After the reaction was completed, 167 g of chlorinated p-benzoquinone was obtained by filtration, with a yield of 93.3%.
Embodiment 2
[0032] Embodiment 2: the preparation of chlorinated p-benzoquinone.
[0033] All the steps in this example are the same as in Example 1, except that 200mL of 10% dilute nitric acid was used to obtain 178g of chlorinated p-benzoquinone, with a yield of 99.4%.
Embodiment 3
[0034] Embodiment 3: the preparation of chlorinated p-benzoquinone.
[0035] In a 1000mL airtight four-necked reaction flask, add a mixture of 800g cyclohexane and 197.5g 2,4-dichlorophenol and 3,4-dichlorophenol, stir to dissolve, and then in a 2000mL airtight four-necked reaction flask , add 50mL of 5% dilute nitric acid and 0.1 mole of cuprous chloride, start to drop the dissolved mixture solution of 2,4-dichlorophenol and 3,4-dichlorophenol, add dropwise 2 moles of hydrogen peroxide, stir and control the temperature for 30 °C, reacted for 1.5 h, and the reaction was completed, and 175 g of chlorinated p-benzoquinone was obtained by filtration, with a yield of 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com