Ethylene antagonist and preparation method thereof
An antagonist and ethylene technology, applied in the field of ethylene antagonists and its preparation, can solve the problems of adverse effects on the environment and equipment, human injury, large amount of by-product waste acid, etc., to avoid corrosive hydrochloric acid gas and high yield Effects on economy, reduction of environmental and equipment hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
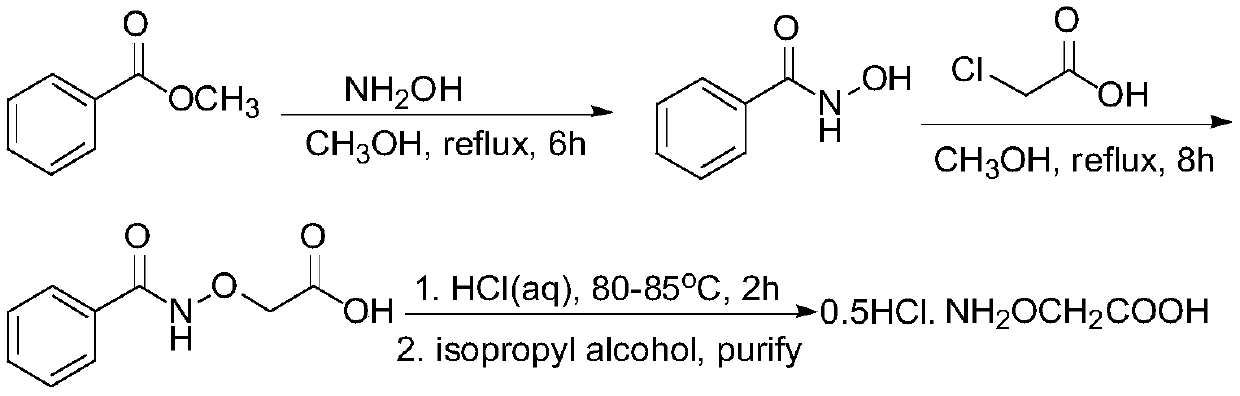
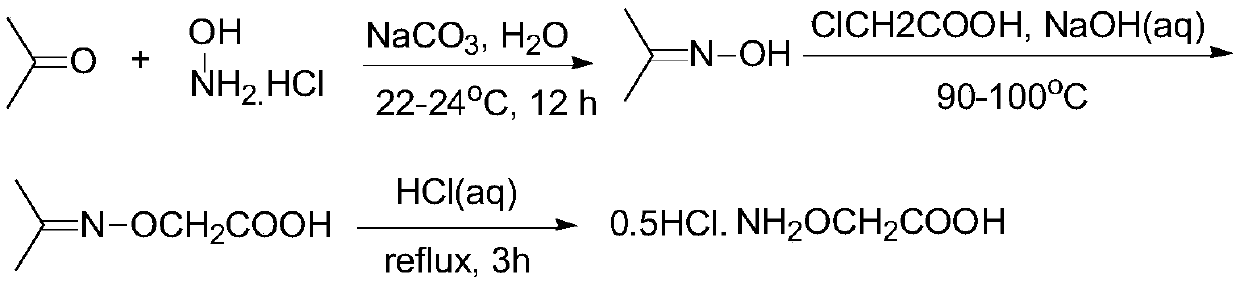
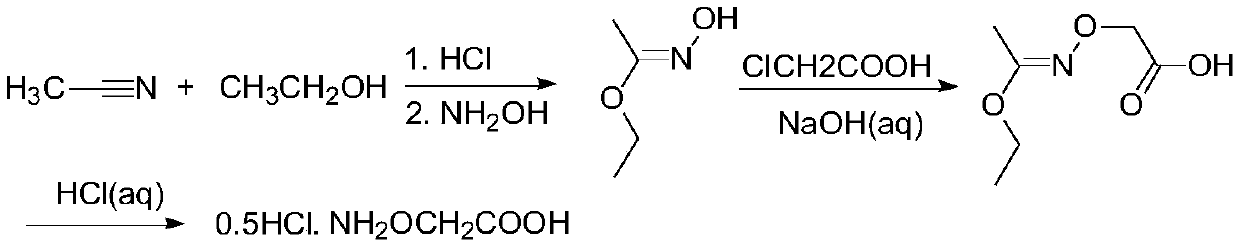
[0033] The preparation method of an ethylene antagonist provided by the invention is obtained through the reaction of isopropylidene aminooxyacetic acid and non-volatile acid.
[0034] The specific preparation method is: heating isopropylidene aminooxyacetic acid in an aqueous solution of a non-volatile acid to remove the protective group to generate a salt of aminooxyacetic acid, and detect when the residual amount of isopropylidene aminooxyacetic acid in the raw material is <0.1%. It is the end point of the reaction; the obtained product aqueous solution is concentrated under reduced pressure to remove part of the water; then an alcoholic organic solvent is added to crystallize the salt, and cooled and stirred to complete the crystallization; then the solvent is removed by filtration, and the filter cake is washed with an alcoholic solvent and dried to obtain the final product .
Embodiment 1
[0036] Add 26 grams (0.2 moles) of isopropylidene aminooxyacetic acid into 200 ml of 1 mol / liter sulfuric acid aqueous solution and heat to reflux at 100°C for 15 hours, (the residual amount of isopropylidene aminooxyacetic acid in the raw material is detected <0.1%) , and then the reaction solution was concentrated under reduced pressure to 50 ml. Then 200 milliliters of isopropanol was added under stirring while hot, and a large amount of white crystals could be observed to separate out. The temperature of the system was lowered to 0°C-5°C, stirred and crystallized for 2 hours, then filtered, and the filter cake was washed with 40 ml of cold isopropanol, and vacuum-dried to obtain 25.2 g of aminooxyacetic acid sulfate (90% molar yield). The molar ratio of aminooxyacetic acid to sulfate in the obtained sulfate is 2:1, that is, one molecule of sulfate can form a salt with two molecules of aminooxyacetic acid. The reaction equation is as follows:
[0037]
Embodiment 2
[0039] 26 grams (0.2 moles) of isopropylidene aminooxyacetic acid was added to 200 milliliters of 1.5 mol / liter phosphoric acid aqueous solution and heated to reflux at 80°C for 20 hours (the residual amount of isopropylidene aminooxyacetic acid in the raw material was detected <0.1%), Then the reaction solution was concentrated under reduced pressure to 60 mL. Then, 200 milliliters of isopropanol was added under stirring while hot, and a large amount of white crystals could be observed to separate out. The temperature of the system was lowered to 0°C-5°C, stirred and crystallized for 2 hours, then filtered, and the filter cake was washed with 40 ml of cold isopropanol, and then vacuum-dried to obtain 33.6 g of aminooxyacetic acid phosphate (89% molar yield). The molar ratio of aminooxyacetic acid to phosphate in the obtained phosphate is 1:1, that is, one molecule of phosphate can form a salt with one molecule of aminooxyacetic acid. The reaction equation is as follows:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com