A kind of non-precious metal oxygen reduction electrocatalyst and its preparation method and application
An electrocatalyst, non-precious metal technology, used in circuits, electrical components, battery electrodes, etc., can solve problems such as insufficient activity and reduced catalyst active site density, and achieve the effect of high oxygen reduction performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
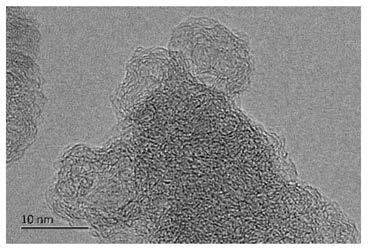
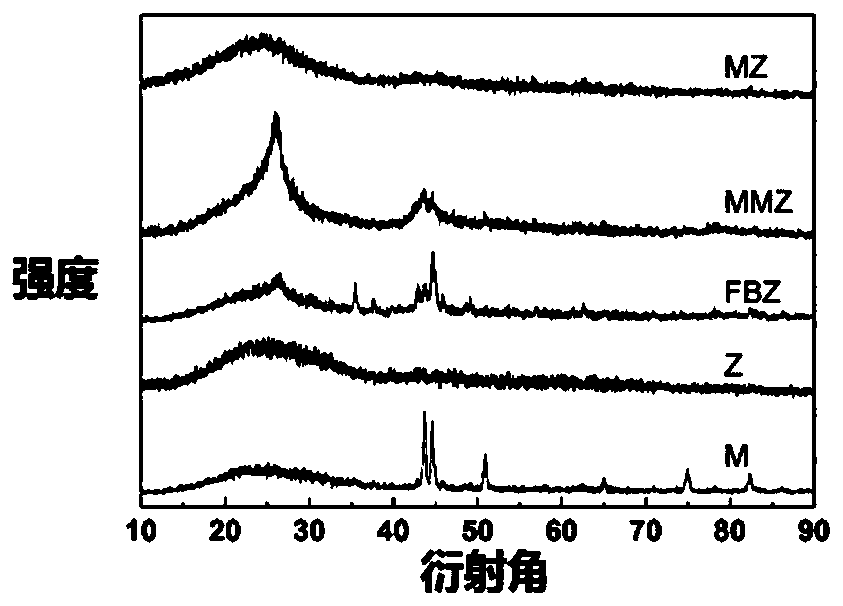
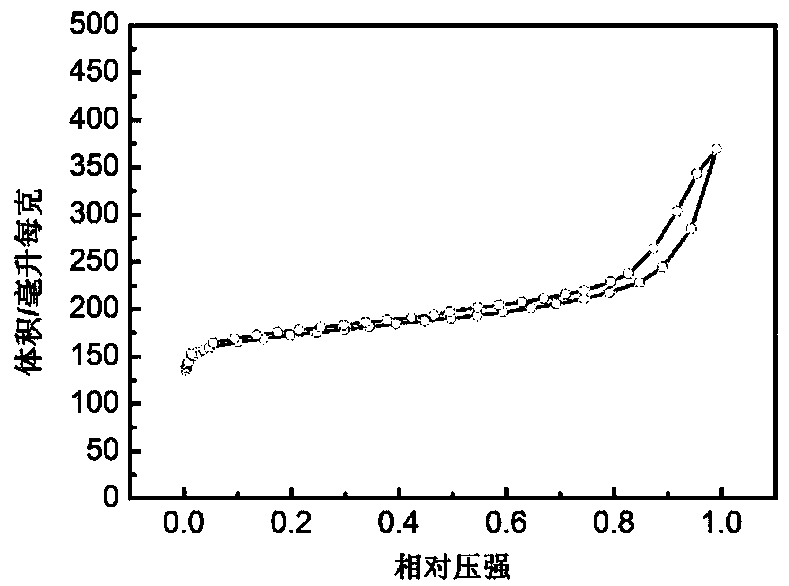
[0032] Weigh 2.03g ferric chloride hexahydrate in a beaker, add 25ml DMF, stir to form a solution, weigh 0.618g terephthalic acid in a beaker, add 25ml DMF, stir to form a solution, slowly pour the ferric chloride solution into the terephthalic acid solution, continue to stir for 30 minutes, then transfer to the reactor and heat to 110 ° C for 8 hours. After the reaction, the reactor is cooled to room temperature, and the product is obtained by centrifugation, and washed with ethanol three times to remove the residual solvent. Thus MIL-101(Fe) was obtained. At 20°C, 1.470g of zinc nitrate hexahydrate and 3.260g of 2-methylimidazole were dissolved in 50ml of methanol respectively, and the former was slowly added to the latter under stirring, continued to stir for 12min, and then stood still for 20h. Centrifuge, wash three times, and dry in vacuum at 150° C. for 8 hours to obtain ZIF-8. Mix MIL-101(Fe) and ZIF-8 at a mass ratio of 1:10 and ball mill for 8h, then transfer to a c...
Embodiment 2
[0035] Weigh 2.03g ferric chloride hexahydrate in a beaker, add 25ml DMF, stir to form a solution, weigh 0.618g terephthalic acid in a beaker, add 25ml DMF, stir to form a solution, slowly pour the ferric chloride solution into the terephthalic acid solution, continue to stir for 30 minutes, then transfer to the reactor and heat to 110 ° C for 8 hours. After the reaction, the reactor is cooled to room temperature, and the product is obtained by centrifugation, and washed with ethanol three times to remove the residual solvent. Thus MIL-101(Fe) was obtained. At 20°C, 1.470g of zinc nitrate hexahydrate and 3.260g of 2-methylimidazole were dissolved in 50ml of methanol respectively, and the former was slowly added to the latter under stirring, continued to stir for 12min, and then stood still for 20h. Centrifuge, wash three times, and dry in vacuum at 150° C. for 8 hours to obtain ZIF-8. Mix MIL-101(Fe) and ZIF-8 at a mass ratio of 1:5 and ball mill for 8h, then transfer to a co...
Embodiment 3
[0038] Weigh 2.03g ferric chloride hexahydrate in a beaker, add 25ml DMF, stir to form a solution, weigh 0.618g terephthalic acid in a beaker, add 25ml DMF, stir to form a solution, slowly pour the ferric chloride solution into the terephthalic acid solution, continue to stir for 30 minutes, then transfer to the reactor and heat to 110 ° C for 8 hours. After the reaction, the reactor is cooled to room temperature, and the product is obtained by centrifugation, and washed with ethanol three times to remove the residual solvent. Thus MIL-101(Fe) was obtained. At 20°C, 1.470g of zinc nitrate hexahydrate and 3.260g of 2-methylimidazole were dissolved in 25ml of methanol respectively, and the former was slowly added to the latter under stirring, continued to stir for 12min, and then stood still for 20h. Centrifuge, wash three times, and dry in vacuum at 150° C. for 8 hours to obtain ZIF-8. Mix MIL-101(Fe) and ZIF-8 at a mass ratio of 1:10 and ball mill for 8h, then transfer to a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com